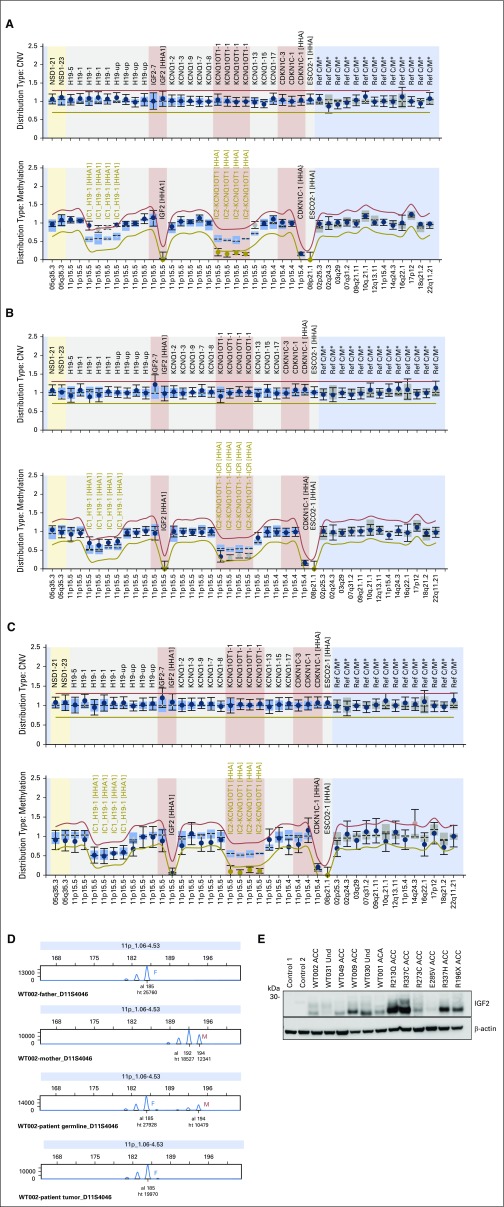
Fig A1.
Chromosome 11p15 abnormalities as visualized by methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) and microsatellite analysis in DNA from pediatric patients with adrenocortical tumors (ACTs) without TP53 mutations. (A) MS-MLPA analysis of WT031 blood DNA showing (upper panel) normal copy number of chromosome 11p15 and (lower panel) gain of methylation at imprinting control region 1 (IC1) with loss of methylation at imprinting control region 2 (IC2) indicating uniparental disomy. (B) WT002 blood DNA showing partial gain of methylation at IC1 and partial loss of methylation at IC2. (C) MS-MLPA analysis of WT023 blood DNA showing loss of methylation at IC2. (D) Representative microsatellite marker analysis of parents (blood) and patient (blood and tumor) DNA. The father (F) is homozygous (185/185) and the mother (M) heterozygous (192/194) for the D11S4046 marker. Patient blood DNA shows inheritance of alleles 185 from father and 194 from mother; (bottom panel) allele 194 (maternal origin) is selected against in the tumor. (E) Western blot analysis performed with 50 µg of protein with goat polyclonal antihuman antibody directed against insulin-like growth factor 2 (IGF2; 1:500 dilution; Sigma-Aldrich, St Louis, MO) as previously reported.23 β-actin (1:2,000; Sigma-Aldrich) was used as the loading control. This analysis included patients with 11p15 abnormalities on germline (n = 6) and with germline-mutated TP53, as indicated. Levels of IGF2 were higher in samples than in control (two normal adrenocortical tissues obtained during nephrectomy for Wilms tumor) and were not affected by genotype (wild-type or mutated TP53) or histology (carcinoma, adenoma, or undetermined [Und]). ACA, adrenocortical adenoma; ACC, adrenocortical carcinoma; CNV, copy number variation; al, allele; ht, height.