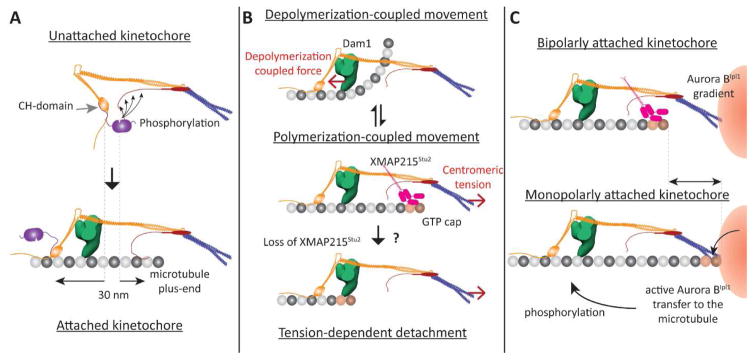
Figure 3. Proposed architecture-function relationships for the yeast kinetochore (1-D representations of the kinetochore shown).
A Role of kinetochore architecture in SAC inactivation: Separation of the CH-domains of Ndc80 and the phosphodomain of KNL1Spc105 by end-on attachment (highlighted by dashed lines) disrupts the phosphorylation of KNL1Spc105 by the Mps1 kinase (magenta) bound to the CH-domain. B Proposed roles of the architecture of microtubule-binding proteins in generating bidirectional movement. Top: When the plus-end is depolymerizing, the Dam1 ring (green) mechanically opposes the curling of tubulin protofilaments, and experiences a pushing force (red arrow). Middle panel: We propose that XMAP215Stu2 localizes to the kinetochore by recognizing the GTP-tubulin cap on the polymerizing plus-end. Its microtubule-destabilizing activity reverts the plus-end back to the depolymerizing state. Bottom panel: In the absence of XMAP215 activity, centromeric tension can slide the kinetochore off the growing plus-end. C Potential roles for kinetochore architecture in correcting monopolar attachment: The position of the plus-end may be significantly different in kinetochores with bipolar and monopolar attachment (highlighted by the arrow, top and bottom panels respectively). The proximity of the lattice to the centromere may facilitate the transport of hyper-activated Aurora BIpl1 kinase to its targets – microtubule-binding kinetochore proteins.