Fig. 1.
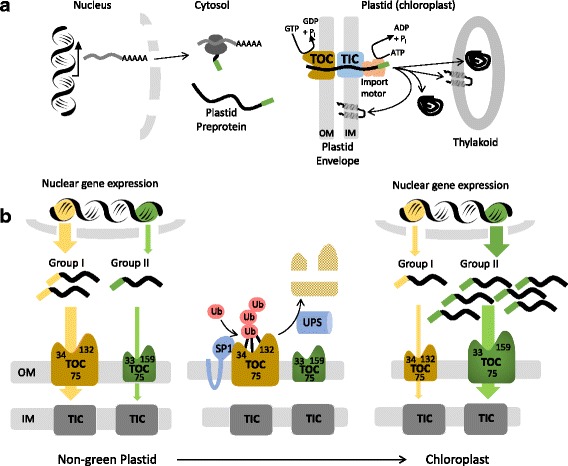
Plastid protein import and control of the import machinery by the ubiquitin-proteasome system. a The majority of plastid proteins are encoded in the nucleus and translated on cytosolic ribosomes. Plastid preproteins contain an N-terminal transit peptide that is necessary and sufficient to target proteins to the organelle. The transit peptide is recognized at the surface of the plastid by two GTPase receptors of the TOC complex (brown), Toc159 (159) and Toc33 (33), at the outer envelope membrane (OM). The receptors initiate membrane transport via a GTP-dependent switch, and the preprotein translocates through an associated β-barrel channel, Toc75 (75) of the TOC complex. Import occurs simultaneously across TOC and TIC (blue) and is driven by an ATP-dependent import-associated chaperone network, which constitutes the import motor (orange). The transit peptide is removed by the stromal processing peptidase upon import, and the chaperone network assists in folding and assembly of the newly imported proteins. Proteins destined for the inner envelope or thylakoid membranes are subsequently recognized by conserved sub-organellar targeting machineries. b Distinct TOC complexes (brown and green), defined by the presence of specific TOC GTPase receptors (e.g., Toc159/33 vs. Toc132/34) mediate import of specific classes of preproteins, thereby preventing competition for import between proteins from different functional or developmental-specific groups (e.g., Groups I and II) and providing a mechanism of selectively regulating their import. The turnover of TOC complexes plays a key role in plastid-type transitions, including the conversion from chemoautotrophic to photoautotrophic metabolism in seedlings. TOC complex turnover is controlled by the ubiquitin proteasome system (UPS) via an outer envelope-associated RING-type E3 ubiquitin ligase, SP1. This functions to balance the levels of specific TOC pathways with changes in the expression of specific classes of preproteins to maintain organelle homeostasis
