Fig. 2.
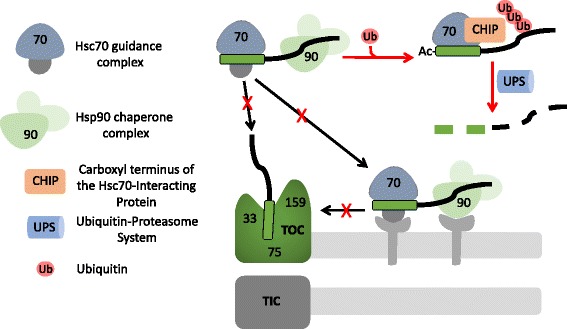
Targeting of preproteins to the TOC complex is monitored by the ubiquitin proteasome system in the cytosol. Preproteins are targeted to the TOC complex post-translationally as unfolded polypeptides, and cells must monitor protein import to avoid the toxic accumulation of mis-sorted or misfolded preproteins in the cytosol. This is particularly critical during plastid developmental transitions when TOC complexes are turned over, or under stress conditions when import is inhibited. Cytosolic Hsp70 (blue) and Hsp90 (green) chaperones have been implicated as components of targeting complexes to assist preprotein transit through the cytoplasm en route to their interaction with the TOC receptors (black arrows). When import of preproteins is inhibited (red X), an Hsp70 isoform, Hsc70-4, functions in conjunction with the cytosolic E3 ubiquitin ligase, CHIP (orange), to target misfolded or mis-sorted preproteins for degradation by the cytosolic ubiquitin-proteasome system (UPS; red arrow). N-terminal acetylation is prevalent under conditions that result in the accumulation of preproteins in the cytosol, suggesting that this might serve as a marker for UPS-mediated degradation
