Fig. 3.
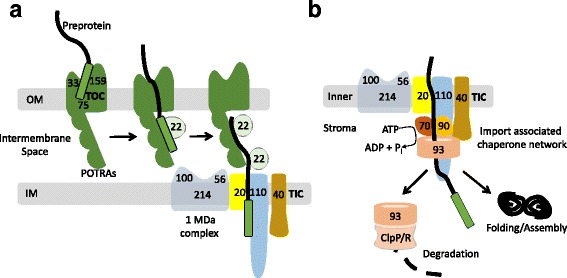
Chaperone systems in the intermembrane space and stroma assist cooperation between TOC and TIC and provide the driving force for import. a Import across the outer and inner envelope membranes (OM and IM) through TOC and TIC is coupled to provide direct targeting from the cytosol to the plastid stroma. Toc75 (75), the major membrane channel of the TOC complex (green), contains three polypeptide transport associated domains (POTRAs) that bind to preproteins in the intermembrane space as they emerge across the outer envelope. The POTRAs and Tic22 (light green), an intermembrane space chaperone, work together to ensure that preproteins do not misfold in the intermembrane space and assist in hand-off to the TIC machinery. In some species, preprotein targeting to the TIC system is facilitated by a 1 MDa complex at the inner membrane (gray) that includes Tic56 (56), Tic100 (100) and Tic214 (214). Tic20 (20), Tic110 (110), and Tic40 (40) are major components of the translocation machinery at the inner membrane. b Membrane translocation is driven by an import-associated chaperone network, containing cpHsp70 (70), cpHsp90 (90), and Hsp93/ClpC (93), which assemble at the site of import by the coordinate actions of Tic110 (110) and Tic40 (40). This chaperone network functions as an ATP-dependent import motor and may assist in folding and assembly of newly imported proteins. Recent evidence also suggests that Hsp93/ClpC is associated with the ClpP/R protease (ClpP/R), leading to the hypothesis that the Clp complex functions as a quality control system to degrade newly imported proteins that are orphaned or misfolded
