Abstract
Background
The timing of mitral valve surgery in asymptomatic patients with primary mitral regurgitation (MR) is controversial. We hypothesized that the forward left ventricular (LV) ejection fraction (LVEF; ie, LV outflow tract stroke volume divided by LV end‐diastolic volume) is superior to the total LVEF to predict outcomes in MR. The objective of this study was to examine the association between echocardiographic parameters of MR severity and LV function and outcomes in patients with MR.
Methods and Results
The clinical and Doppler‐echocardiographic data of 278 patients with ≥mild MR and no class I indication of mitral valve surgery at baseline were retrospectively analyzed. The primary study end point was the composite of mitral valve surgery or death. During a mean follow‐up of 5.4±3.2 years, there were 147 (53%) events: 96 (35%) MV surgeries and 66 (24%) deaths. Total LVEF and global longitudinal strain were not associated with the occurrence of events, whereas forward LVEF (P<0.0001) and LV end‐systolic diameter (P=0.0003) were. After adjustment for age, sex, MR severity, Charlson probability, coronary artery disease, and atrial fibrillation, forward LVEF remained independently associated with the occurrence of events (adjusted hazard ratio: 1.09, [95% confidence interval]: 1.02–1.17 per 5% decrease; P=0.01), whereas LV end‐systolic diameter was not (P=0.48).
Conclusions
The results of this study suggest that the forward LVEF may be superior to the total LVEF and LV end‐systolic diameter to predict outcomes in patients with primary MR. This simple and easily measurable parameter may be useful to improve risk stratification and select the best timing for intervention in patients with primary MR.
Keywords: ejection fraction, longitudinal strain, mitral valve regurgitation, risk stratification
Subject Categories: Echocardiography
Clinical Perspective
What Is New?
Forward left ventricular (LV) ejection fraction (LVEF) is a composite marker of mitral regurgitation (MR) severity and LV systolic dysfunction, which is superior to total LVEF, LV end‐systolic diameter, or global longitudinal strain to predict outcomes in patients with primary MR.
Patients with a forward LVEF <50% are at higher risk for adverse events. A preoperative forward LVEF <40% was associated with increased risk of LV systolic dysfunction after mitral valve surgery.
What Are the Clinical Implications?
Forward LVEF is a simple parameter to calculate from routine echocardiography.
Forward LVEF may be useful to improve risk stratification and trigger mitral valve surgical intervention before LV dysfunction becomes irreversible in patients with severe MR.
A reduced forward LVEF in patients with mild or moderate MR should lead to closer echocardiographic follow‐up and/or additional tests to corroborate MR severity.
Forward LVEF could be very helpful in identifying the patients potentially eligible for earlier mitral valve repair with minimally invasive techniques.
Introduction
Mitral regurgitation (MR) is the most frequent valvular disease in Western countries.1 Surgical or transcatheter mitral valve (MV) repair or replacement are the only available therapies for severe MR and are associated with improved prognosis and survival.2 However, the optimal timing of MV surgery in asymptomatic patients with severe MR remains debated.3, 4, 5, 6, 7, 8, 9, 10, 11 In the 2016 American College of Cardiology/American Heart Association12 and 2017 European Society of Cardiology/European Association for Cardio‐Thoracic Surgery13 guidelines, the only Class I indication for the realization of MV surgery in patients with severe MR is the presence of symptoms and/or left ventricular (LV) systolic dysfunction. In the guidelines, LV systolic dysfunction is defined as an LV ejection fraction <60% and/or an LV end‐systolic diameter ≥4012 or ≥45 mm.13 However, several studies suggest that, in patients with severe MR, MV surgery performed before the onset of symptoms or LV systolic dysfunction is associated with better survival compared to a “watchful waiting” strategy.3, 4, 5, 6, 7 Hence, there is a need to improve individualized risk stratification and therapeutic decision making in MR.
A decline in LV ejection fraction (LVEF) or an increase in LV end‐systolic diameter is associated with worse prognosis in patients with MR.14, 15, 16 However, because of the presence of MR, the “total” LVEF measured by the Simpson's method may grossly underestimate the extent of intrinsic myocardial systolic dysfunction.17 Hence, potentially irreversible myocardial dysfunction may develop insidiously despite maintained preserved LVEF and absence of symptoms. We hypothesized that the forward LVEF calculated by the Dumesnil method18 is more sensitive than total LVEF to detect subclinical LV dysfunction and predict events. The objective of this study was thus to examine the impact of the echocardiographic parameters of MR severity and LV systolic function on outcomes in patients with primary MR.
Methods
Study Population
The clinical and Doppler‐echocardiographic data of 278 consecutive patients with ≥mild primary MR were retrospectively analyzed. Exclusion criteria were: (1) >mild mitral stenosis, mild aortic regurgitation, or mild aortic stenosis, (2) Class I indication for MV surgery (ie, severe MR with symptoms and/or LV systolic dysfunction), and (3) history of MV replacement or repair. Patients requiring an MV surgery within 6 months following baseline echocardiography were excluded.
The study was approved by the Ethics Committee of the Quebec Heart and Lung Institute, and written informed consent was waived for this retrospective analysis.
Clinical Data
Clinical data, including age, sex, weight, height, cardiovascular risk factors, comorbidities, and current medications, were collected. Charlson probability of 10 years mortality was calculated for each patient at baseline.19
Doppler Echocardiographic Data
Doppler echocardiographic measurements were performed according to recommendations of the American Society of Echocardiography20 and included: MR severity, LV dimensions, left atrial dimensions, LV forward stroke volume (SV) measured in the LV outflow tract using pulsed wave Doppler, total and forward LVEF, and global longitudinal strain (GLS). LV ejection index (indexed LV end‐systolic diameter divided by LV outflow tract time‐velocity integral)21 was also calculated.
MR severity
The etiology of MR was prolapse in 99% and mal‐coaptation in 1%. The MR jet(s) was assessed by color Doppler echocardiography in multiple windows, and MR severity was graded into 3 classes using a multiparametric integrative approach as recommended in the guidelines22: mild, moderate, or severe. The parameters of MR severity were MR vena contracta width, effective regurgitation orifice area, regurgitant volume and MR regurgitant fraction calculated by proximal isovelocity area method and/or by the volumetric method when available, pressure half time of MR jet velocity, and flow reversal pattern in the pulmonary veins.
LV systolic function
We calculated the total and forward LVEFs. Total LVEF was calculated by the biplane Simpson's method:
where LV end‐diastolic and end‐systolic volumes were measured by the biplane Simpson's method.
The forward LVEF was calculated by the Dumesnil method (Figure 1)18:
where the forward SV is measured by pulsed wave Doppler in the LV outflow tract and LV end‐diastolic volume is calculated from the LV end‐diastolic diameter by the Teichholz formula23:
Figure 1.
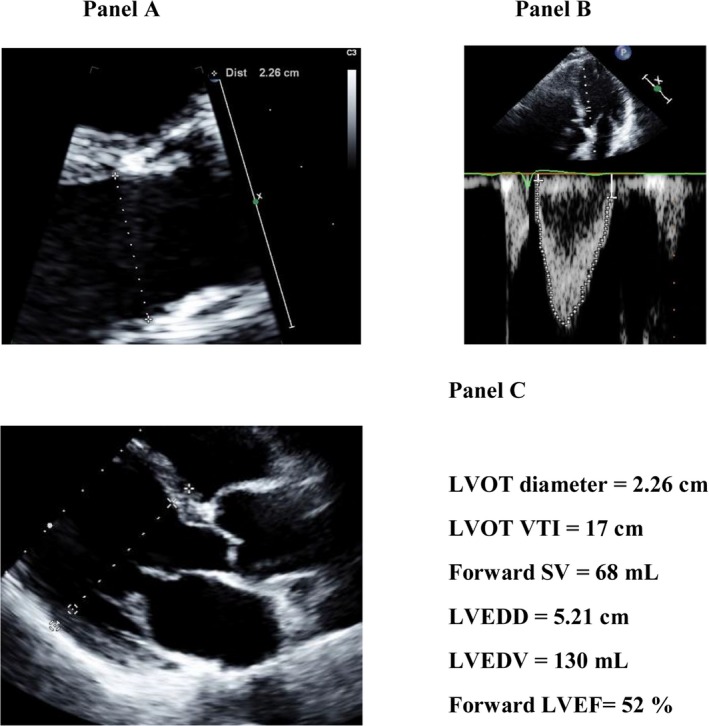
Calculation of the forward LVEF by the Dumesnil method. The forward LVEF is calculated by dividing the forward LV SV measured in the LV outflow tract from the LV outflow tract diameter (A) and flow velocity‐time integral (B) by the LV end‐diastolic volume calculated from the LV end‐diastolic diameter (C) by the LV indicates left ventricular; LVEDD, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; SV, stroke volume; VTI, velocity‐time integral.
The intra‐ and interobserver percent variability for the measurement of total LVEF were respectively 3.6% and 4.4% and the measurement of forward LVEF was 5.1% for both (intra and inter). The intraclass coefficient for intra‐ and interobserver agreement was 0.82 and 0.78, respectively, for total LVEF and 0.98 and 0.97 for forward LVEF. The measurements of LV GLS were performed retrospectively by the speckle‐tracking method on a dedicated workstation (Cardiac Performance Analysis; TomTec Imaging Systems, Munich, Germany). GLS was measured on the apical 2‐, 3‐, and 4‐chamber views and averaged. GLS measurements were available in 158 patients (57%). The main reasons for missing GLS measurement were nonavailability or poor quality (low frame rate, poor echogenicity) of echocardiographic images, because of the retrospective design. Intra‐ and interobserver variability for the GLS measures was 6.2% and 6.8%, respectively. Intraclass coefficient was 0.94 for intraobserver and 0.94 for interobserver measurements of GLS.
Trans‐tricuspid peak systolic pressure gradient was calculated from tricuspid regurgitation peak velocity using the Bernoulli equation.
Study End Points
The primary end point of this study was the occurrence of the composite of MV surgery or death. Patients were referred for MV surgery because of the occurrence of symptoms, LV systolic dysfunction, pulmonary hypertension, and/or atrial fibrillation. The secondary end points were: (1) all‐cause mortality and (2) all‐cause mortality under conservative management. The information of the timing and type of MV surgery was retrospectively collected from patients' chart. Mortality data were obtained from the central database of the Quebec Institute of Statistics.
Statistical Analysis
Continuous variables are expressed as mean±SD and categorical variables as percentage. Results of the Cox proportional hazards analyses are presented as hazard ratio (HR) and 95% confidence interval (95% CI). All variables in the Cox models verified the proportional hazards assumption based on inspection of trends in the Schoenfeld residuals (all P≥0.11).
Receiver operating characteristic curve analyses were used to assess the sensitivity, specificity, and positive and negative predictive values for various thresholds of each independent predictor to determine outcomes. Kaplan–Meier curves and Wilcoxon tests of the time‐to‐event data were used to assess the impact of MR severity, indexed LV end‐systolic diameter, forward LVEF, indexed LV SV, indexed left atrial diameter, and trans‐tricuspid gradient on primary and secondary end points. To identify the variables associated with the primary and secondary end points, clinically relevant variables and variables with a P<0.10 in univariable Cox proportional hazard analyses were included into the multivariable model. We avoided to include in the same model variables that were strongly inter‐related. Multicollinearity between the variables entered in the models was assessed by variance inflation factor. Variables were considered collinear if the variance inflation factor ≥3. The predictive performance of multivariable models was assessed by comparing likelihood ratios of the models. The incremental value of forward LVEF to predict 2‐year events beyond traditional risk factors was assessed using category‐free net reclassification index. Statistical analyses were performed with JMP software (version 12). P<0.05 was considered statistically significant.
Results
Baseline Characteristics
Table 1 shows the baseline characteristics of the study population. Mean age was 65±15 years, 58% of the patients were males, 53% had diagnosis of hypertension, 7% diabetes mellitus, 38% dyslipidemia, 28% had a history of coronary artery disease, and atrial fibrillation was found in 22% of the cohort. Mean Charlson probability index was 0.56±0.35. MR severity was mild in 105 patients (38%), moderate in 117 patients (42%), and severe in 56 patients (20%; Table 2). Forward LVEF calculated by the Dumesnil method was 53±16%, total LVEF calculated by Simpson's method was 65±5%, and GLS was −21.2±−2.8% (Table 2). Forward LVEF decreased with increasing MR severity (P<0.0001), whereas total LVEF was similar across all MR severity classes (P=0.61; Figure 2). Sixteen patients were lost to follow‐up. However, from the Quebec Institute of Statistic database, we were able to confirm that these patients were still alive at the time of the study.
Table 1.
Baseline Clinical Characteristics According to Presence or Absence of Clinical Events
| Variables | All Patients n=278 | No Events n=131 (47%) | Events n=147 (53%) | P Value |
|---|---|---|---|---|
| Age, y | 65.0±15.1 | 62.6±15.8 | 67.1±14.2 | 0.02 |
| Male sex | 160 (58) | 65 (50) | 95 (65) | 0.01 |
| BMI, kg/m² | 23.5±3.6 | 23.4±3.6 | 23.7±3.8 | 0.44 |
| Risk factors | ||||
| Hypertension | 143 (53) | 62 (47) | 81 (55) | 0.25 |
| Diabetes mellitus | 20 (7) | 7 (5) | 13 (9) | 0.28 |
| Obesity | 12 (4) | 6 (5) | 6 (4) | 0.84 |
| Dyslipidemia | 101 (38) | 43 (33) | 58 (39) | 0.28 |
| Current smoking | 28 (11) | 10 (8) | 18 (12) | 0.19 |
| History of smoking | 84 (33) | 39 (30) | 45 (31) | 0.88 |
| Coronary artery disease | 70 (28) | 20 (18) | 50 (36) | 0.001 |
| Atrial fibrillation | 60 (22) | 23 (18) | 37 (25) | 0.13 |
| Charlson probability | 0.56±0.35 | 0.62±0.32 | 0.50±0.36 | 0.02 |
| Medications | ||||
| Statins | 88 (33) | 41 (31) | 47 (32) | 0.92 |
| ACEI | 87 (32) | 28 (22) | 59 (42) | 0.007 |
| ARB | 49 (18) | 25 (19) | 24 (16) | 0.56 |
| β‐blockers | 71 (26) | 30 (23) | 41 (28) | 0.36 |
| Diuretics | 82 (30) | 35 (27) | 47 (32) | 0.32 |
| Nitrates | 23 (9) | 9 (7) | 14 (10) | 0.41 |
| Follow‐up time | 5.4±3.2 | 7.1±2.7 | 3.9±2.8 | <0.0001 |
Values are expressed as mean±SD or n (%). ACE indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; BMI, body mass index.
Table 2.
Baseline Doppler Echocardiographic Data According to Presence or Absence of Clinical Events
| Variables | All Patients n=278 | No Events n=131 (47%) | Events n=147 (53%) | P Value |
|---|---|---|---|---|
| MR grade | <0.0001 | |||
| Mild | 105 (38) | 70 (53) | 35 (24) | |
| Moderate | 117 (42) | 50 (39) | 67 (46) | |
| Severe | 56 (20) | 11 (8) | 45 (31) | |
| MR effective regurgitant orifice area, cm2 | 0.35±0.19 | 0.27±0.14 | 0.43±0.19 | <0.0001 |
| MR regurgitant volume, mL | 55±27 | 45±20 | 66±29 | <0.0001 |
| MR regurgitant fraction, % | 44±12 | 40±11 | 47±13 | 0.0006 |
| LV end‐diastolic diameter, mm | 51±6 | 49±6 | 53±6 | <0.0001 |
| Indexed LV end‐diastolic diameter, mm/m2 | 29±3 | 28±3 | 30±3 | 0.002 |
| LV end‐systolic diameter, mm | 31±6 | 29±5 | 31±6 | 0.007 |
| Indexed LV end‐systolic diameter, mm/m2 | 17±3 | 17±3 | 18±4 | 0.07 |
| Forward LV stroke volume, mL | 64±16 | 66±17 | 62±15 | 0.02 |
| Indexed forward LV stroke volume, mL/m2 | 36±8 | 38±8 | 35±8 | 0.002 |
| LV cardiac output, L/min | 4.40±1.19 | 4.45±1.14 | 4.36±1.23 | 0.54 |
| LV shortening fraction, % | 41±9 | 41±8 | 41±9 | 0.93 |
| LVEF, % | ||||
| Forward LVEF (%) | 53±16 | 59±15 | 47±15 | <0.0001 |
| Total LVEF, % | 65±5 | 65±4 | 65±6 | 0.86 |
| LV global longitudinal strain, % | −21.22±2.83 | −21.26±2.44 | −21.18±3.26 | 0.86 |
| LA diameter, mm | 42±8 | 39±6 | 44±8 | <0.0001 |
| Indexed LA diameter, mm/m2 | 24±5 | 23±4 | 25±5 | <0.0001 |
| Indexed LA volume, mL/m² | 37±17 | 33±11 | 42±20 | 0.0002 |
| Trans‐tricuspid gradient, mm Hg | 30±10 | 28±9 | 31±11 | 0.006 |
LA indicates left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; MR, mitral regurgitation.
Figure 2.
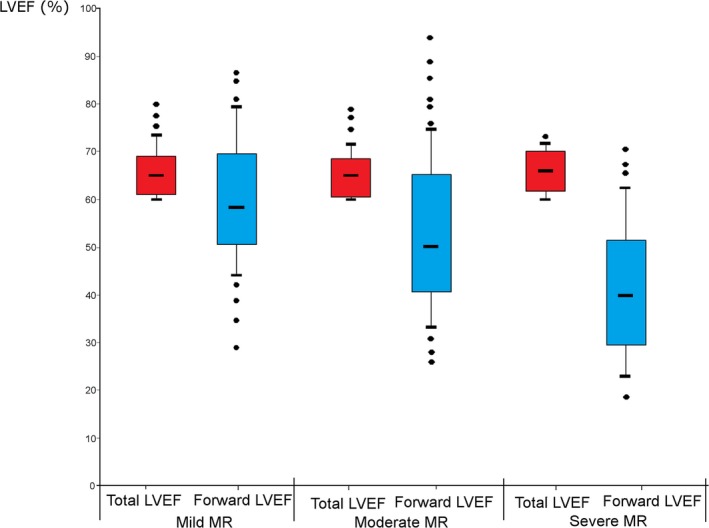
Comparison of total LVEF and forward LVEF according to MR severity. This figure shows the comparison of the total LVEF vs the forward LVEF according to the MR severity grade at baseline. LVEF indicates left ventricular ejection fraction; MR, mitral regurgitation.
Factors Associated With the Composite of MV Surgery or Death
During a mean follow‐up of 5.4±3.2 years, there were 147 (53%) primary end points: 96 MV surgeries and 51 deaths. Furthermore, there were 15 postoperative deaths. Among the 105 patients with mild MR at baseline, 46 progressed to moderate MR and 20 to severe MR during follow‐up. Among the 117 patients with moderate MR at baseline, 58 progressed to severe MR during follow‐up. Seventeen patients with mild MR and 49 with moderate MR required a MV surgery during the follow‐up.
Patients who experienced an event (MV surgery or death) during follow‐up were older, had more‐severe MR at baseline, and more comorbidities than those without events. There was no association between heart rate or arterial pressure and occurrence of events. In univariable analysis, higher values of indexed LA diameter (P<0.0001), LV end‐diastolic diameter (P<0.0001), LV end‐systolic diameter (P=0.0003) and trans‐tricuspid pressure gradient (P=0.002), and lower forward LVEF (P<0.0001) and indexed LV forward SV (P=0.0003) were associated with occurrence of MV surgery or death (Figure 3). Patients with a forward LVEF <50% (HR, 2.47; 95% CI, 1.76–3.50; P<0.0001) had at least 2‐fold increased risk of MV surgery or death compared with patients with higher forward LVEF. Total LVEF (P=0.49) and GLS (P=0.50) were not associated with events (Table S1).
Figure 3.
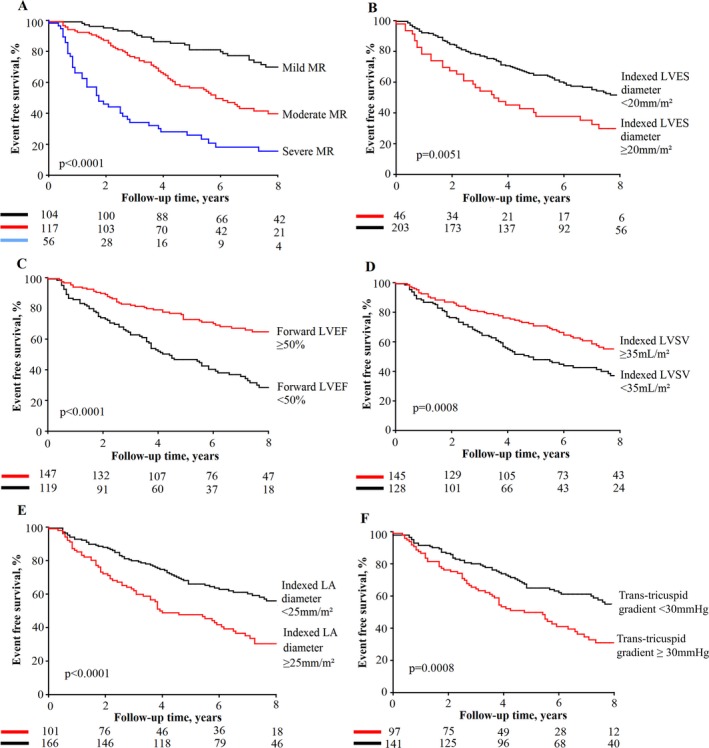
Event‐free survival in patients with MR according to baseline echocardiographic parameters. This figure shows the event‐free survival curves for the composite of MV surgery or death according to various echocardiographic parameters of MR severity and LV function: MR grade (A), indexed LVES diameter (B); forward LVEF (C); indexed LVSV (D); Indexed LA diameter (E); trans‐tricuspid gradient (F). LA indicates left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; LVES, left ventricular end‐systolic; LVSV, left ventricular stroke volume; MR, mitral regurgitation; MV, mitral valve.
On multivariable analysis, after adjustment for age, sex, MR severity, Charlson probability, coronary artery disease, treatment with angiotensin‐converting enzyme inhibitors, and atrial fibrillation, forward LVEF (HR, 1.09; 95% CI, 1.02–1.17 per 5% absolute decrease; P=0.01) and indexed LA diameter (HR, 1.06; 95% CI, 1.02–1.101‐mm/m2 increase; P=0.004) were the variables that had the strongest independent association with outcomes (Table 3). The addition of forward LVEF into the multivariable model, including all variables mentioned above, significantly improved the model (likelihood ratio test, P=0.03). Furthermore, forward LVEF significantly improved the risk classification of patients for clinical events with a net reclassification index of 32% (P=0.04).
Table 3.
Multivariable Predictors of Clinical Events, Mortality, and Mortality Under Conservative Management
| Increment | MV Surgery or Deatha | All‐Cause Mortalityb | Mortality on Medical Treatmentb | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| LV end‐systolic diameter | 5‐mm increase | 1.08 (0.87–1.34) | 0.48 | 1.18 (0.91–1.52) | 0.21 | 1.13 (0.81–1.58) | 0.46 |
| Forward LVEF | 5% decrease | 1.09 (1.02–1.17) | 0.01 | 1.14 (1.05–1.24) | 0.0007 | 1.13 (1.04–1.24) | 0.005 |
| Indexed LA diameter | 1‐mm/m2 increase | 1.06 (1.02–1.10) | 0.004 | 1.05 (0.99–1.11) | 0.11 | 1.04 (0.97–1.11) | 0.28 |
| Trans‐tricuspid gradient | 5‐mm Hg increase | 1.01 (0.90–1.13) | 0.86 | 0.99 (0.86–1.14) | 0.87 | 0.97 (0.82–1.15) | 0.76 |
ACE indicates angiotensin‐converting enzyme inhibitors; CI, confidence interval; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MV, mitral valve.
Adjusted for: age, sex, MR severity, Charlson probability, coronary artery disease, atrial fibrillation, and ACE inhibitors.
Adjusted for: age, sex, MR severity, Charlson probability, and mitral valve surgery.
In the subset of 174 patients with ≥moderate MR at baseline, forward LVEF was independently associated with clinical events (HR, 1.13; 95% CI, 1.05–1.21 per 5% decrease; P=0.0007) in a multivariable model with the same adjustment as described above.
When building a multivariable model including LV ejection index instead of forward LVEF, this index was not associated with clinical events (HR, 1.36; 95% CI, 0.73–2.37; P=0.32). Association with events was stronger with forward LVEF than with LV ejection index, and the likelihood ratio of the model including forward LVEF was significantly better (P=0.02) than that of the model including the LV ejection index.
Factors Associated With All‐Cause Mortality
Sixty‐six patients died during the follow‐up; 23 from a cardiovascular condition, 19 from cancer, 6 from a pulmonary condition, 4 from a gastrointestinal condition, 4 from a renal condition, and 10 from an unknown cause. Fifteen patients died after an MV surgery.
Patients who died during follow‐up were older and had more comorbidities and more medications. In univariable analysis, larger indexed end‐systolic LV diameter and left atrial diameter, lower indexed LV SV, higher trans‐tricuspid gradient, and absence of MV surgery were associated with increased risk of mortality (all P<0.01; Figure S1). Forward LVEF, entered in continuous or dichotomized (>/≤50%) format, was strongly associated with all‐cause mortality (both P=0.008). There was a trend toward an association between lower total LVEF and mortality (P=0.10).
After adjustment for age, sex, Charlson probability, MR severity, and MV surgery entered as a time‐dependent variable, the forward LVEF was independently associated with increased mortality (HR, 1.14; 95% CI, 1.05–1.24 per 5% decrease; P=0.0007; Table 3). Adjustment for indexed LV end‐systolic diameter instead of LV end‐systolic diameter in the multivariable model provided similar results. Further adjustment for total LVEF did not modify the results: Total LVEF was not associated with mortality (P=0.11), whereas forward LVEF remained independently associated with mortality (P=0.002).
In the subset of patients in whom GLS was available (n=158, 57%), GLS was associated with increased all‐cause mortality (P=0.004). In multivariable analysis adjusted for age, sex, and Charlson probability, forward LVEF (HR, 1.14; 95% CI, 1.01–1.29 per 5% decrease; P=0.04) was independently associated with overall mortality in this subset of patients. In a separate model including the same patients, adjusted for the same variables, GLS was also associated with all‐cause mortality (HR, 2.32; 95% CI, 1.10–5.04 per 5% decrease in absolute value; P=0.03). Comparison of the likelihood ratio of the 2 models demonstrated no significant difference regarding their respective impact on all‐cause mortality (P=0.42). Further analysis adjusted for the same variables and the same patients, but including total LVEF instead of forward LVEF or GLS, showed no significant association between total LVEF and all‐cause mortality (HR, 1.43; 95% CI, 0.92–2.22 per 5% decrease; P=0.11).
In a multivariable model including LV ejection index instead of forward LVEF, LV ejection index was independently associated with overall mortality (HR, 2.04; 95% CI, 1.01–3.79 per 5 mL/m2 decrease; P=0.05). The association with overall mortality was similar for forward LVEF and LV ejection index; likelihood ratio between the 2 models were comparable (P=0.78).
Factors Associated With All‐Cause Mortality Under Conservative Management
During a mean follow‐up of 5.4±3.2 years, 51 patients (18%) died under conservative management. In univariable analysis, severe MR, larger indexed end‐systolic LV diameter, left atrial diameter, higher trans‐tricuspid pressure gradient, lower indexed LV forward SV, forward LVEF, and GLS were significantly associated with excess mortality on conservative management (all P<0.01), whereas total LVEF showed no significant impact (P=0.19; Figure S2; Table S1).
In multivariable analysis adjusted for age, sex, Charlson probability, and MR severity, forward LVEF (HR, 1.13; 95% CI, 1.04–1.24 per 5% decrease; P=0.005) remained independently associated with all‐cause mortality under conservative management (Table 3). Adjustment for indexed LV end‐systolic diameter instead of LV end‐systolic diameter in the multivariable model provided similar results.
Association Between Total LVEF or Forward LVEF and Postoperative LV Dysfunction
Figure S3 shows the changes in total LVEF, forward LVEF, and GLS following MV surgery. Among the 96 patients who underwent MV surgery during follow‐up, 27 (28%) had LV systolic dysfunction (total LVEF <50%) within the first 6 months after operation and 20 (21%) had LV systolic dysfunction beyond 6 months after operation. After adjustment for age and sex, lower preoperative forward LVEF was significantly associated with the occurrence of postoperative LV dysfunction (odds ratio, 1.51; 95% CI, 1.07–2.30 per 5% absolute decrease in LVEF; P=0.02), whereas preoperative total LVEF (P=0.14) or LV end‐systolic diameter (P=0.50) were not. The cut‐off value of preoperative forward LVEF providing the best accuracy for predicting postoperative LV systolic dysfunction (ie, total LVEF <50%) was <40% (sensitivity, 82%; specificity=61%). Following MV surgery, the forward LVEF increased by 12±1%, whereas total LVEF decreased by 10±1%.
The addition of preoperative forward LVEF into a multivariable model including age, sex, preoperative total LVEF, and preoperative LV end‐systolic diameter significantly improved the predictive value of the model for postoperative LV dysfunction (likelihood ratio test, P=0.05).
Discussion
The findings of this study suggest that the forward LVEF, which can easily be calculated by the Dumesnil method (Figure 1), compares favorably with the parameters of LV systolic function currently proposed in the guidelines, that is, total LVEF and LV end‐systolic diameter, to predict outcomes in patients with primary MR. Furthermore, compared with total LVEF, forward LVEF better predicted the occurrence of postoperative LV systolic dysfunction in the patients who underwent mitral valve surgery.
In patients with severe MR, surgery is almost unavoidable, and the remaining question is more about the timing of the intervention. Some studies support an earlier “prophylactic” intervention,3, 4, 5, 6, 7 whereas others support a watchful waiting strategy with intervention at the onset of symptoms and/or LV systolic dysfunction.8, 9, 10, 11 The 2016 American College of Cardiology/American Heart Association updated guidelines for management of patients with primary MR recommend surgery for symptomatic patients or asymptomatic patients with LVEF <60% or LV end‐systolic diameter ≥40 mm (Class I).12
Enriquez‐Sarano et al24 reported that long‐term survival is almost 2‐fold lower in patients with severe MR operated for Class I indication (heart failure symptoms, LVEF <60%, and/or LV end‐systolic diameter ≥40 mm) than asymptomatic patients with severe MR operated earlier in the absence of these criteria. Moreover, as reported in previous studies and further confirmed in the present study, even a moderate MR is associated with worse outcomes compared with mild MR. This finding may be related to the following factors: (1) Patients with moderate MR may rapidly progress to severe MR during follow‐up; (2) a large proportion (up to one third) of patients with moderate MR at rest develop severe MR and pulmonary hypertension during exercise25, 26; and (3) subclinical myocardial dysfunction may develop early in the course of MR well before reaching the hemodynamically severe stage.27, 28
The challenge in patients with asymptomatic MR is to detect LV systolic dysfunction at an early or subclinical stage so that surgical correction can be instituted to prevent the development of irreversible dysfunction. The deterioration in LVEF is often not preceded by the occurrence of symptoms or atrial fibrillation, and when it occurs it is often irreversible following surgery. LV dysfunction may thus develop insidiously in the asymptomatic patient and may become irreversible.29, 30 The 2 sole parameters of LV function that are presented in the guidelines to trigger MV intervention in severe MR are: a total LVEF <60% and an LV end‐systolic diameter ≥40 mm (or ≥45 mm in the European Society of Cardiology/European Association for Cardio‐Thoracic Surgery guidelines).12, 13 However, these parameters may lack sensitivity and there is a need to develop more‐sensitive markers of subclinical LV dysfunction. Total LVEF and LV end‐systolic dimensions may indeed underestimate the degree of actual LV systolic dysfunction in the presence of significant MR. Moreover, the more severe is the MR, the greater is the underestimation of LV dysfunction by total LVEF or LV systolic diameter (Figure 4). The GLS has the potential to better reflect the intrinsic myocardial function compared with total LVEF in MR.31, 32, 33 However, this parameter may also be influenced, to some extent, by MR severity and loading conditions. Hence, the GLS may actually be more sensitive than total LVEF, but less sensitive than the forward LVEF, for early detection of myocardial systolic dysfunction (Figure 3 and 4).
Figure 4.
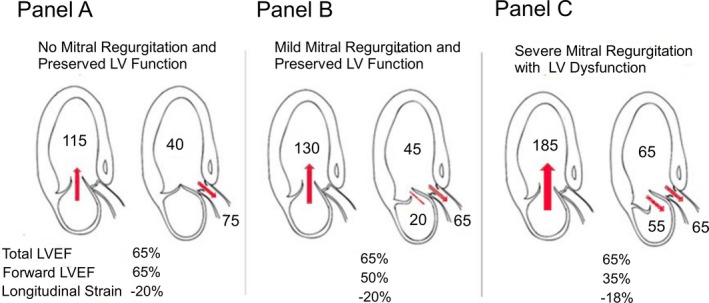
Comparison between total LVEF, forward LVEF and global longitudinal strain according to MR severity and associated LV dysfunction. This figure shows the comparison of Total LVEF, Forward LVEF and Global Longitudinal Strain when there is no mitral regurgitation (A), a mild mitral regurgitation with preserved LV systolic function (B), and a severe mitral regurgitation with a LV systolic dysfunction (C). LV indicates left ventricular; LVEF, left ventricular ejection fraction; MR, mitral regurgitation.
In 1985, Clancy et al suggested that the forward LVEF, that is, the ratio of the forward SV to the LV end‐diastolic volume, measured by cardiac catheterization may provide a useful index of LV function in patients with MR and preserved LVEF.34 In the present study, we found that the forward LVEF is the parameter of LV systolic function that showed the strongest association with the occurrence of clinical events (ie, MV surgery or death). Hence, these findings suggest that, although it does not reflect the true LVEF, the forward LVEF is more sensitive than the total LVEF to detect subclinical LV dysfunction and predict risk of adverse events in primary MR. Consistent with our results, Magne et al recently reported that the “LV ejection index,” calculated by dividing the indexed LV end‐systolic diameter by LV outflow tract time‐velocity integral, was a better predictor of mortality and LV dysfunction after surgery than total LVEF.21 Our results, however, suggest that forward LVEF may be superior to the LV ejection index to predict outcomes in primary MR, especially the need of MV surgery. Several studies15, 29, 35 reported that a substantial proportion of patients may have a significant deterioration of total LVEF immediately after MV surgery. This finding was often interpreted as an acute deterioration in the intrinsic myocardial function attributed to increase in LV afterload related to the correction of MR. However, this acute decline in myocardial function may not be entirely real and may be, at least in part, the reflection of the actual state of LV function before the surgery, whereas total LVEF grossly underestimates the extent of LV systolic dysfunction before surgery because of the confounding effect of the mitral regurgitant volume. To this effect, Gelfand et al36 found that there is a significant correlation and agreement between preoperative forward LVEF and postoperative total LVEF. In the present study, the forward LVEF measured before MV surgery was superior to total LVEF to predict the occurrence of LV systolic dysfunction after surgery. Hence, the forward LVEF might be superior to the total LVEF to predict the LVEF after correction of MR. Clancy et al34 also reported that patients with forward LVEF <35% had higher short‐term mortality following MV surgery in patients with severe MR. Further prospective studies are needed to confirm the incremental prognostic value of forward LVEF beyond the standard parameters of LV systolic function in patients with MR.
Clinical Implications
The apparent lack of clinical equipoise attributed to the recent publication of several studies3, 4, 5, 6, 7 supporting early prophylactic surgery makes very difficult the realization of a randomized, controlled trial comparing the 2 treatment strategies: that is, early surgery versus watchful waiting. On the other hand, the generalization of 1 strategy or the other to all patients with asymptomatic severe MR would likely yield to suboptimal management and outcome of a substantial proportion of these patients.11, 37
With the generalization of early surgery, there would be a risk of overtreatment or treatment too early in the course of the disease, whereas with the generalization of the watchful waiting, there would be a risk of treatment too late with ensuing permanent cardiovascular and functional sequel. Individualized risk stratification and therapeutic decision making is probably the best approach to manage patients with primary MR. The main concern in patients with asymptomatic moderate or severe MR is the risk of subclinical and potentially irreversible LV dysfunction. The forward LVEF is a simple parameter to calculate from the routine echocardiography and it is superior to the total LVEF, the LV systolic diameter, or the GLS to predict outcomes in patients with primary MR. Furthermore, the inter‐ and intraobserver reproducibility of forward LVEF appears to be better than that of total LVEF. In patients with asymptomatic severe MR, this parameter may be helpful to trigger surgical intervention before LV dysfunction becomes irreversible. A forward LVEF <50% was associated with a 2.47‐fold increase in the composite of MV surgery or death in the present study. Furthermore, a preoperative forward LVEF <40% was associated with increased risk of LV systolic dysfunction after MV surgery. In patients with mild or moderate MR, the finding of a reduced forward LVEF should lead to closer echocardiographic follow‐up and/or additional tests (ie, exercise stress echo) to corroborate the MR severity and identify the presence of other causes (coronary artery disease, hypertension, etc) to explain the LV dysfunction.
The development of new minimally invasive and off‐cardiopulmonary bypass surgical techniques38, 39, 40 may allow durable mitral valve repair with very low surgical risk. These techniques could thus enhance the perspective and feasibility of earlier intervention in patients with asymptomatic severe MR. The assessment of the forward LVEF could be very helpful in identifying the patients potentially eligible for earlier MV repair with these new minimally invasive techniques.
Limitations
This study was an observational, retrospective study and may have selection bias. We elected to also include patients with mild or moderate MR because, as well illustrated in this study, MR is a disease that may progress rapidly, and moderate or even mild MR is not necessarily benign in all patients. Furthermore, there is a paucity of data on the echocardiographic parameters that may help to identify patients who are at higher risk of rapid disease progression and occurrence adverse events in patients with mild/moderate MR. Echocardiographic exams were performed and read by different sonographers and cardiologists, and there was no centralized reading in a core laboratory. The study sample size was relatively small and the limited number of deaths prevented us to perform a comprehensive multivariable analysis including all potentially relevant variables in the in the same model. Moreover, because of the small number of cardiac deaths, it was not possible to perform separate multivariable analyses for this end point. The cardiovascular medications may change the LV preload and afterload and therefore influence the total or forward LVEF. However, in the present study, there was no significant association between the different medications and the forward or total LVEF.
Conclusion
This study shows that forward LVEF, that can be easily calculated by the Dumesnil method, is strongly and independently associated with adverse events, that is, MV surgery or death, in patients with primary MR. Furthermore, the forward LVEF compares favorably with the standard echocardiographic parameters, that is, total LVEF calculated by Simpson's method and LV end‐systolic diameter, to assess LV systolic function and trigger MV surgery in MR. Patients with a forward LVEF <50% are at higher risk for adverse events. These findings suggest that forward LVEF may be taken into account for the risk stratification and therapeutic decision making in patients with primary MR. Further prospective studies are needed to confirm the prognostic value and clinical utility of the forward LVEF in these patients.
Sources of Funding
This work was supported by a grant from Canadian Institutes of Health Research, Ottawa, Ontario, Canada (MOP# 102737 and FDN‐ 143225). Dupuis was supported by a studentship grant from Canadian Institutes of Health Research and a studentship grant from the Quebec Heart and Lung Institute. Clavel was supported by a postdoctoral fellowship grant from Canadian Institutes of Health Research. Dahou was supported by a fellowship grant from L'Agence de la santé et des services sociaux de la Capitale Nationale, Québec, Québec, Canada. Thébault was supported by a clinical and research fellowship grant from the Fédération Française de Cardiologie. Arsenault is a research scholar from the Fonds de Recherche en Santé du Québec (FRSQ), Montreal, Québec, Canada. Pibarot holds the Canada Research Chair in Valvular Heart Diseases, Canadian Institutes of Health Research.
Disclosures
None.
Supporting information
Table S1. Univariable Predictors of Clinical Events, Mortality, and Mortality Under Conservative Management
Figure S1. Overall survival in patients with MR according to baseline echocardiographic parameters.
Figure S2. Survival under conservative management in patients with MR according to baseline echocardiographic parameters.
Figure S3. Total LVEF, forward LVEF, and GLS before and following MV surgery.
(J Am Heart Assoc. 2017;6:e006309 DOI: 10.1161/JAHA.117.006309.)29079561
References
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 2. Detaint D, Sundt TM, Nkomo VT, Scott CG, Tajik AJ, Schaff HV, Enriquez‐Sarano M. Surgical correction of mitral regurgitation in the elderly: outcomes and recent improvements. Circulation. 2006;114:265–272. [DOI] [PubMed] [Google Scholar]
- 3. Enriquez‐Sarano M, Sundt TM III. Early surgery is recommended for mitral regurgitation. Circulation. 2010;121:804–811. [DOI] [PubMed] [Google Scholar]
- 4. Enriquez‐Sarano M, Avierinos JF, Messika‐Zeitoun D, Detaint D, Capps M, Nkomo V, Scott C, Schaff HV, Tajik AJ. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–883. [DOI] [PubMed] [Google Scholar]
- 5. Kang DH, Park SJ, Sun BJ, Cho EJ, Kim DH, Yun SC, Song JM, Park SW, Chung CH, Song JK, Lee JW, Park PW. Early surgery versus conventional treatment for asymptomatic severe mitral regurgitation: a propensity analysis. J Am Coll Cardiol. 2014;63:2398–2407. [DOI] [PubMed] [Google Scholar]
- 6. Kang DH, Kim JH, Rim JH, Kim MJ, Yun SC, Song JM, Song H, Choi KJ, Song JK, Lee JW. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation. 2009;119:797–804. [DOI] [PubMed] [Google Scholar]
- 7. Suri RM, Vanoverschelde JL, Grigioni F, Schaff HV, Tribouilloy C, Avierinos JF, Barbieri A, Pasquet A, Huebner M, Rusinaru D, Russo A, Michelena HI, Enriquez‐Sarano M. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA. 2013;310:609–616. [DOI] [PubMed] [Google Scholar]
- 8. Rosenhek R. Watchful waiting for severe mitral regurgitation. Semin Thorac Cardiovasc Surg. 2011;23:203–208. [DOI] [PubMed] [Google Scholar]
- 9. Rosenhek R, Rader F, Klaar U, Gabriel H, Krejc M, Kalbeck D, Schemper M, Maurer G, Baumgartner H. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation. 2006;113:2238–2244. [DOI] [PubMed] [Google Scholar]
- 10. Gillam LD, Marcoff L, Shames S. Timing of surgery in valvular heart disease: prophylactic surgery vs watchful waiting in the asymptomatic patient. Can J Cardiol. 2014;30:1035–1045. [DOI] [PubMed] [Google Scholar]
- 11. Gillam LD, Schwartz A. Primum non nocere: the case for watchful waiting in asymptomatic “severe” degenerative mitral regurgitation. Circulation. 2010;121:813–821. [DOI] [PubMed] [Google Scholar]
- 12. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 2017;70:252–289. [DOI] [PubMed] [Google Scholar]
- 13. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Muñoz DR, Rosenhek R, Sjögren J. 2017 ESC/EACTS guidelines for the management of valvular heart disease: The Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2017. [Google Scholar]
- 14. Dujardin KS, Seward JB, Orszulak TA, Schaff HV, Bailey KR, Tajik AJ, Enriquez‐Sarano M. Outcome after surgery for mitral regurgitation. Determinants of postoperative morbidity and mortality. J Heart Valve Dis. 1997;6:17–21. [PubMed] [Google Scholar]
- 15. Enriquez‐Sarano M, Tajik AJ, Schaff HV, Orszulak TA, McGoon MD, Bailey KR, Frye RL. Echocardiographic prediction of left ventricular function after correction of mitral regurgitation: results and clinical implications. J Am Coll Cardiol. 1994;24:1536–1543. [DOI] [PubMed] [Google Scholar]
- 16. Enriquez‐Sarano M, Tajik AJ, Schaff HV, Orszulak TA, Bailey KR, Frye RL. Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation. 1994;90:830–837. [DOI] [PubMed] [Google Scholar]
- 17. Gaasch WH, John RM, Aurigemma GP. Managing asymptomatic patients with chronic mitral regurgitation. Chest. 1995;108:842–847. [DOI] [PubMed] [Google Scholar]
- 18. Dumesnil JG, Dion D, Yvorchuk K, Davies RA, Chan K. A new, simple and accurate method for determining ejection fraction by Doppler echocardiography. Can J Cardiol. 1995;11:1007–1014. [PubMed] [Google Scholar]
- 19. Charlson M, Pompei P, Ales K, MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 20. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. [DOI] [PubMed] [Google Scholar]
- 21. Magne J, Szymanski C, Fournier A, Malaquin D, Avierinos JF, Tribouilloy C. Clinical and prognostic impact of a new left ventricular ejection index in primary mitral regurgitation because of mitral valve prolapse. Circ Cardiovasc Imaging. 2015;8:e003036. [DOI] [PubMed] [Google Scholar]
- 22. Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, Hagendorff A, Monin JL, Badano L, Zamorano JL. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr. 2010;11:307–332. [DOI] [PubMed] [Google Scholar]
- 23. Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic‐angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11. [DOI] [PubMed] [Google Scholar]
- 24. Enriquez‐Sarano M, Suri RM, Clavel MA, Mantovani F, Michelena HI, Pislaru S, Mahoney DW, Schaff HV. Is there an outcome penalty linked to guideline‐based indications for valvular surgery? Early and long‐term analysis of patients with organic mitral regurgitation. J Thorac Cardiovasc Surg. 2015;150:50–58. [DOI] [PubMed] [Google Scholar]
- 25. Magne J, Lancellotti P, Piérard LA. Exercise‐induced changes in degenerative mitral regurgitation. J Am Coll Cardiol. 2010;56:300–309. [DOI] [PubMed] [Google Scholar]
- 26. Magne J, Lancellotti P, Piérard LA. Exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. Circulation. 2010;122:33–41. [DOI] [PubMed] [Google Scholar]
- 27. Debonnaire P, Delgado V, Bax JJ. Potential role of fibrosis imaging in severe valvular heart disease. Heart. 2015;101:397–407. [DOI] [PubMed] [Google Scholar]
- 28. Maniar HS, Brady BD, Lee U, Cupps BP, Kar J, Wallace KM, Pasque MK. Early left ventricular regional contractile impairment in chronic mitral regurgitation occurs in a consistent, heterogeneous pattern. J Thorac Cardiovasc Surg. 2014;148:1694–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suri RM, Schaff HV, Dearani JA, Sundt TM III, Daly RC, Mullany CJ, Sarano ME, Orszulak TA. Determinants of early decline in ejection fraction after surgical correction of mitral regurgitation. J Thorac Cardiovasc Surg. 2008;136:442–447. [DOI] [PubMed] [Google Scholar]
- 30. Suri RM, Schaff HV, Dearani JA, Sundt TM, Daly RC, Mullany CJ, Enriquez‐Sarano M, Orszulak TA. Recovery of left ventricular function after surgical correction of mitral regurgitation caused by leaflet prolapse. J Thorac Cardiovasc Surg. 2009;137:1071–1076. [DOI] [PubMed] [Google Scholar]
- 31. Witkowski TG, Thomas JD, Debonnaire PJ, Delgado V, Hoke U, Ewe SH, Versteegh MI, Holman ER, Schalij MJ, Bax JJ, Klautz RJ, Marsan NA. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur Heart J Cardiovasc Imaging. 2013;14:69–76. [DOI] [PubMed] [Google Scholar]
- 32. Mascle S, Schnell F, Thebault C, Corbineau H, Laurent M, Hamonic S, Veillard D, Mabo P, Leguerrier A, Donal E. Predictive value of global longitudinal strain in a surgical population of organic mitral regurgitation. J Am Soc Echocardiogr. 2012;25:766–772. [DOI] [PubMed] [Google Scholar]
- 33. Lancellotti P, Cosyns B, Zacharakis D, Attena E, Van Camp G, Gach O, Radermecker M, Piérard LA. Important of left ventricular longitudinal function and functional reserve in patients with degenerative mitral regurgitation: assessment by 2‐D speckle tracking. J Am Soc Echocardiogr. 2008;21:1331–1336. [DOI] [PubMed] [Google Scholar]
- 34. Clancy KF, Hakki AH, Iskandrian AS, Hadjimiltiades S, Mundth ED, Hakki AH, Bemis CE, Nestico PF, DePace NL, Segal BL. Forward ejection fraction: a new index of left ventricular function in mitral regurgitation. Am Heart J. 1985;110:658–664. [DOI] [PubMed] [Google Scholar]
- 35. Gaasch WH, Zile MR. Left ventricular function after surgical correction of chronic mitral regurgitation. Eur Heart J. 1991;12(B):48–51. [DOI] [PubMed] [Google Scholar]
- 36. Gelfand EV, Haffajee JA, Hauser TH, Yeon SB, Goepfert L, Kissinger KV, Delatorre R, Manning WJ. Predictors of preserved left ventricular systolic function after surgery for chronic organic mitral regurgitation: a prospective study. J Heart Valve Dis. 2010;19:43–50. [PubMed] [Google Scholar]
- 37. Borer JS. Early surgery or watchful waiting for asymptomatic severe degenerative mitral regurgitation: is the answer now clear? J Am Coll Cardiol. 2014;63:2408–2410. [DOI] [PubMed] [Google Scholar]
- 38. Seeburger J, Rinaldi M, Nielsen SL, Salizzoni S, Lange R, Schoenburg M, Alfieri O, Borger MA, Mohr FW, Aidietis A. Off‐pump transapical implantation of artificial neo‐chordae to correct mitral regurgitation: the TACT Trial (Transapical Artificial Chordae Tendinae) proof of concept. J Am Coll Cardiol. 2014;63:914–919. [DOI] [PubMed] [Google Scholar]
- 39. Colli A, Manzan E, Rucinskas K, Janusauskas V, Zucchetta F, Zakarkaite D, Aidietis A, Gerosa G. Acute safety and efficacy of the NeoChord procedure†. Interact Cardiovasc Thorac Surg. 2015;20:575–580; discussion, 580–581. [DOI] [PubMed] [Google Scholar]
- 40. Gammie JS, Wilson P, Bartus K, Gackowski A, Hung J, D'Ambra MN, Kolsut P, Bittle GJ, Szymanski P, Sadowski J, Kapelak B, Bilewska A, Kusmierczyk M, Ghoreishi M. Transapical beating‐heart mitral valve repair with an expanded polytetrafluoroethylene cordal implantation device: initial clinical experience. Circulation. 2016;134:189–197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariable Predictors of Clinical Events, Mortality, and Mortality Under Conservative Management
Figure S1. Overall survival in patients with MR according to baseline echocardiographic parameters.
Figure S2. Survival under conservative management in patients with MR according to baseline echocardiographic parameters.
Figure S3. Total LVEF, forward LVEF, and GLS before and following MV surgery.


