Abstract
Background
Topical NSAIDs have less systemic absorption than oral NSAIDs. We examined the risk of cardiovascular events associated with nonselective topical NSAIDs versus oral NSAIDs among patients with rheumatoid arthritis in Taiwan.
Methods and Results
We conducted a retrospective cohort study that included patients with incident rheumatoid arthritis who were newly starting therapy with nonselective topical NSAIDs or oral NSAIDs. We used the Taiwan National Health Insurance Research Database (NHIRD). The first date patients received either type of NSAID was defined as the index date. NSAID exposures continued until there was a treatment gap of >30 days. The main outcome was composite cardiovascular events, including myocardial infarction, unstable angina, heart failure, stroke, or revascularization. Follow‐up was censored at treatment discontinuation, switch or addition of other NSAID category, cardiovascular outcome, death, or the end of the study. Propensity score weighted Cox regression models were used to compare the risk of cardiovascular events between topical NSAIDs and oral NSAIDs. There were 10 758 and 78 056 treatment episodes for topical and oral NSAIDs identified. After weighting by propensity score, the cohorts were well balanced over all covariates. The crude cardiovascular event rate was 1.87 per 100 person‐years for topical NSAIDs and 2.14 per 100 person‐years for oral NSAIDs. Results of propensity score weighted Cox regression found the topical NSAID group had 36% lower risk for cardiovascular events compared with the oral NSAID group (hazard ratio, 0.64; 95% confidence interval, 0.43–0.95).
Conclusions
We found topical NSAID users experienced a reduced risk of cardiovascular events compared with oral NSAID users. If future studies with a larger sample size and longer follow‐up confirm these results, NSAID prescribing might change accordingly.
Keywords: cardiovascular outcomes, comparative effectiveness, NSAIDs, pharmacoepidemiology, rheumatoid arthritis
Subject Categories: Cardiovascular Disease, Epidemiology, Quality and Outcomes
Clinical Perspective
What Is New?
We found Taiwanese patients with rheumatoid arthritis who were receiving topical nonselective NSAIDs experienced a reduced risk of cardiovascular events compared with those receiving oral nonselective NSAIDs, and the results are consistent in patients with preexisting cardiovascular diseases.
What Are the Clinical Implications?
The US Food and Drug Administration strengthened the label warnings for the risk of cardiovascular events with all NSAID use, regardless of the routes of administration and preexisting cardiovascular diseases.
Topical NSAIDs may provide symptom relief without associated systemic adverse events because of favorable pharmacokinetic and pharmacodynamic properties, and we found topical nonselective NSAIDs may be a safer alternative for relieving muscle‐skeletal pain in patients with cardiovascular diseases.
Patients with rheumatoid arthritis (RA) have had a 1.5‐ to 2.0‐fold higher risk for coronary artery disease, myocardial infarction, and stroke than the general population,1, 2 and they commonly receive NSAIDs for symptom relief. In recent years, studies have found that oral NSAIDs are associated with increased cardiovascular risks,3, 4, 5 leading to special concerns of NSAID safety in the population with RA. A recent meta‐analysis and an observational study found similar increased cardiovascular risk across oral nonselective NSAIDs,6 whereas higher risk was found in selective cyclooxygenase 2 (COX‐2) inhibitors versus oral nonselective NSAIDs.7 However, nonselective NSAIDs were not associated with increased cardiovascular mortality in patients with RA.8
Topical NSAIDs may provide symptom relief without associated systemic adverse events because of favorable pharmacokinetic and pharmacodynamic properties. Topical NSAIDs reach higher local and lower systemic concentration in the body than oral NSAIDs. Topical diclofenac has 5‐ to 17‐fold lower systemic exposure than its oral form.9 Local skin reactions are common adverse events in people taking topical NSAIDs, but they are generally transient and not severe. Topical NSAIDs for musculoskeletal symptoms were at least as efficacious as oral NSAIDs in a recent Cochrane systematic review.10 The follow‐up length in most clinical trials included in the meta‐analysis was <12 weeks,11, 12, 13 which was not sufficient to evaluate the long‐term safety signals, either cardiovascular or renal adverse events. Only a few post hoc analyses compared the cardiovascular risks between topical and oral NSIAIDs. One found topical diclofenac had fewer cardiovascular‐related adverse events than the oral form (1.5% versus 3.5%) during 12 weeks of follow‐up.13
In 2015, the US Food and Drug Administration issued a drug safety communication that strengthened the warning that nonaspirin NSAIDs can cause heart attacks, heart failure, or strokes.14 Specifically, the risk of adverse cardiovascular events can occur as early as the first weeks of using NSAIDs, and can occur in patients with or without heart disease or risk factors for heart disease. Therefore, it is crucial to find a safer alternative for oral NSAIDs, especially for patients with RA who are frequently exposed to and have elevated cardiovascular risks compared with the general population and patients with osteoarthritis. On the basis of the pharmacokinetic/pharmacodynamic profile of the topical NSAIDs and results from a short‐term clinical trial,13 we hypothesize that the long‐term cardiovascular risk in topical nonselective NSAID users may be lower than the risk in oral nonselective NSAID users in typical practice. In this study, we used the national claims data from Taiwan to compare the risk of composite cardiovascular events between topical and oral nonselective NSAIDs in patients with RA.
Methods
Data Source
Data sets were obtained from Taiwan's NHIRD. Taiwan launched a single‐payer NHI program in 1995, and by 2010, 99% of the population was enrolled. The NHIRD contains demographic data of enrollees, information on healthcare professionals and medical facilities, and service records and expenditure claims from inpatient, ambulatory care, and contracted pharmacies for reimbursement purposes. Large computerized databases are provided to scientists in Taiwan for research purposes. Patient identifications in NHIRD were double encrypted and deidentified. This study's protocol was reviewed and approved by the Institutional Review Board of National Cheng Kung University Hospital (Tainan, Taiwan). The requirement for informed consent from patients is waived for studies using NHIRD.
Study Design and Population
A retrospective cohort was constructed and included Taiwanese patients with incident RA (International Classification of Diseases, Ninth Revision [ICD‐9] code: 714.XX, excluding 714.3), aged >18 years, who initiated therapy with a disease‐modifying antirheumatic drug (DMARD) between January 1, 2002 and December 31, 2010. We required these patients to have at least 2 RA diagnoses, 7 days apart, and to receive at least 1 DMARD prescription within 365 days from the first RA diagnosis. The positive predictive value of this algorithm was validated and ranged from 86.2% to 88.9% in the US claims database.15 Furthermore, patients had to receive any topical or oral nonselective NSAID after the first RA diagnosis. The first date patients started topical or oral nonselective NSAID therapy was defined as the index date, and the 6‐month period before the index date was the baseline period for covariate assessment. Patients were excluded if they had any NSAID exposures, cancer, HIV (ICD‐9 code: 042 or V08), psoriatic arthritis (ICD‐9 code: 696), or ankylosing spondylitis (ICD‐9 code: 720) claims or were without complete enrollment information during the baseline period.
Topical or Oral Nonselective NSAID Treatment Episodes
All topical or oral nonselective NSAID prescription records were retrieved from inpatient, outpatient, and pharmacy claims by World Health Organization Anatomical Therapeutic Chemical (ATC) codes. Patients were classified into topical (World Health Organization ATC code: M02AA or D11AX18) or oral (World Health Organization Anatomical Therapeutic Chemical (ATC) code: M01A or M01B, excluding M01AH) nonselective NSAID group based on the NSAID they received on the index date. We confirmed the persistence in each treatment episode of either topical or oral nonselective NSAID by allowing a 30‐day grace period and restricted to a minimum of 15 days of exposure. Patients were eligible to contribute >1 treatment episode if they were free of any NSAIDs for at least 180 days from the end of the last episode. Patients were censored on the last date covered by drugs in each episode, the date switched to or added on the other formulations of nonselective NSAIDs (ie, topical or oral), the date of study outcomes, death, or December 31, 2010.
Outcomes and Covariates
The main study outcome in this study was the composite of cardiovascular events, including myocardial infarction, unstable angina, heart failure, stroke (ischemic, hemorrhagic, and transient ischemic attack), and revascularization (coronary artery bypass graft/percutaneous coronary intervention). Each outcome record was retrieved from the inpatient claims plus corresponding diagnosis (ICD‐9) and/or procedure (Taiwan NHI) codes. We included patient demographic information (age, sex, and income levels), comorbid conditions (diabetes mellitus, hypertension, hyperlipidemia, myocardial infarction, stroke, angina, upper gastrointestinal tract disease, Alzheimer dementia, Parkinson disease, fractures, osteoporosis, liver disease, chronic back pain, gout, and falls), and comedications (proton pump inhibitors, H2‐receptor antagonists, antithrombotic therapy, benzodiazepine, selective serotonin reuptake inhibitor, β blockers, angiotensin‐converting enzyme inhibitor, angiotensin receptor blocker, thiazide diuretics, loop diuretics, oral steroid, and anticonvulsant) that were correlated with cardiovascular diseases (Table S1 provides a detailed variable list and codings).7 These variables plus DMARDs were considered in propensity score (PS) models. We further categorized DMARD exposures into 7 mutually exclusive regimens: methotrexate combination, methotrexate‐free combination, methotrexate only, hydroxychloroquine only, sulfasalazine only, other traditional DMARDs, and any biologic DMARD‐containing regimens. Patient demographic information was determined on the index date, and other covariates were measured within 6 months before each treatment episode.
Statistical Analyses
We used the standardized mean difference to test the differences in baseline covariates between topical and oral nonselective NSAID treatment episodes. Differences >0.1 standardized mean difference (10%) represent a clinically significant difference. Kaplan‐Meier method was used to plot unadjusted survival curves. To better control confounding and preserve statistical power, we calculated the stabilized inverse probability of treatment weights (IPTWs) for each treatment episode and weighted them in the baseline table and Cox proportional hazard regression models, using oral nonselective NSAIDs as the reference. Robust variance estimator was adopted in the Cox models to fix the potential correlations within episodes contributed by the same patients. A PS was calculated for each patient using multivariable logistic regression model conditioning on all covariates included in Table S1. A stabilized IPTW was then developed by multiplying the IPTW in topical and oral nonselective NSAID groups with marginal prevalence of the treatment actually received.16 The mean of the stabilized IPTW was checked to examine outliers and whether the cohort was weighted appropriately.
We conducted sensitivity and subgroup analyses to test the robustness of our study. First, a 1:7 ratio PS greedy matching algorithm was applied to examine our results in a matched cohort.17 Second, although all patients included in our cohort had ever received any DMARD within the first year of RA diagnosis, some NSAID treatment episodes may start before DMARD initiation (eg, started on the first diagnosis code) based on our algorithm for identifying RA cases. We further compared the cardiovascular event risk between topical and oral nonselective NSAIDs in treatment episodes started after DMARD initiation. Third, we extended the grace period for exposures to 60 days. Finally, we examined our results in serial subgroups defined by age, sex, and cardiovascular disease history. All analyses were conducted using SAS version 9.4.
Results
Baseline Characteristics of Topical and Oral Nonselective NSAID Treatment Episodes
There were 46 265 patients with incident RA who met our inclusion criteria, and 46 017 of them (99.5%) received topical or oral NSAIDs after meeting the RA diagnosis. We further identified 10 758 and 78 056 topical and oral NSAID treatment episodes, respectively, from this cohort (Figure 1). Restarting treatment was common; on average, there were 2 episodes per patient during the follow‐up. Topical nonselective NSAID users were older, more frequently had most comorbid conditions (hypertension, stroke, upper gastrointestinal tract disease, fracture, osteoporosis, back pain, osteoarthritis, and chronic renal disease), and used comedications (proton pump inhibitors, antithrombotic therapy, benzodiazepines, angiotensin receptor blockers, and anticonvulsants) compared with oral nonselective NSAID users. More single or combination use of methotrexate and biologic DMARD exposures were observed in topical nonselective NSAID groups (Table 1 and Table S2). Nevertheless, after weighting by IPTW, the weighted cohort showed excellent balance between 2 groups across all covariates (mean of IPTW, 1.00±0.17).
Figure 1.
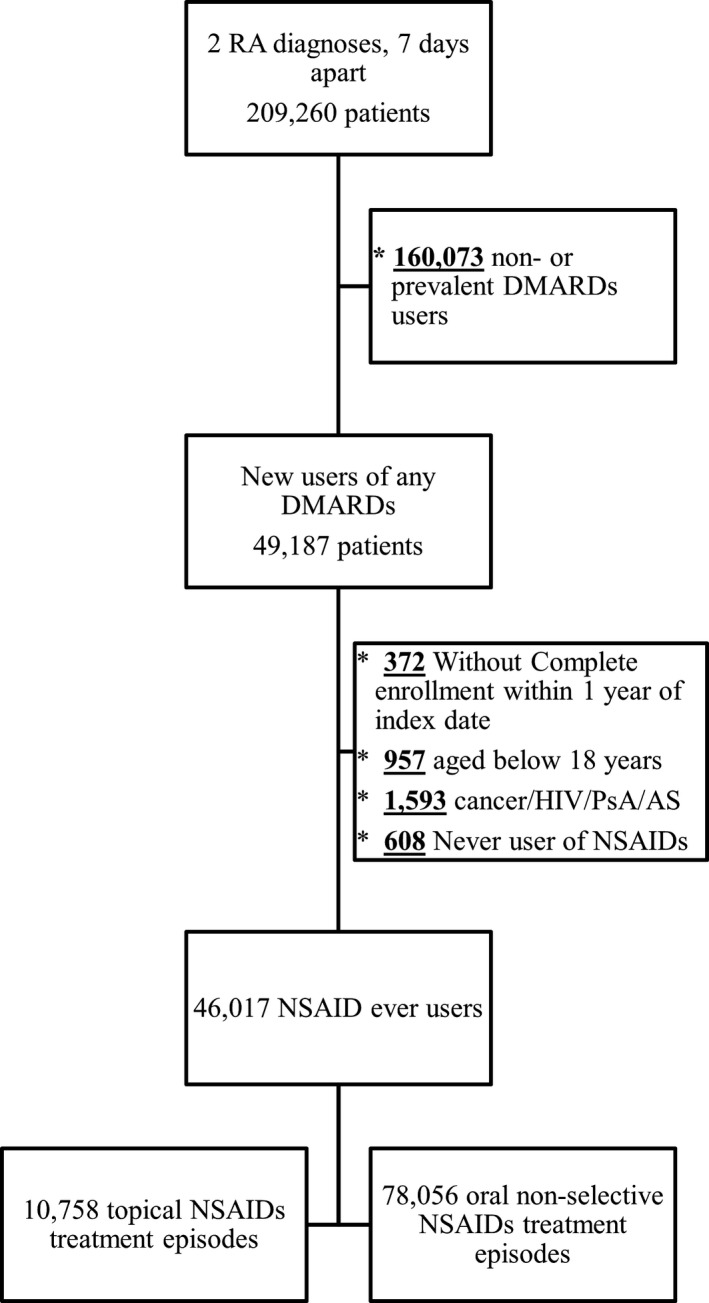
Inclusion flow chart. AS indicates ankylosing spondylitis; DMARD, disease‐modifying antirheumatic drug; PsA, psoriatic arthritis; and RA, rheumatoid arthritis.
Table 1.
Characteristics of Treatment Episodes in the Original and PS Adjusted Cohorts Using Topical or Oral Nonselective NSAIDs
| Characteristics | Original Cohort | IPTW Adjusted | ||||
|---|---|---|---|---|---|---|
| Topical NSAIDs (N=10 758) | Oral NSAIDs (N=78 056) | SMDa | Topical NSAIDs (N=10 687) | Oral NSAIDs (N=78 097) | SMDa | |
| Age, mean (SD), y | 55.1 (15.4) | 51.7 (15.1) | 0.22 | 52.1 (16.0) | 52.1 (15.2) | 0.00 |
| Female sex, % | 82.3 | 76.0 | 0.22 | 77.0 | 76.8 | 0.00 |
| Income, % | 0.10 | 0.01 | ||||
| Low | 3.4 | 3.0 | 3.0 | 3.0 | ||
| Middle | 36.5 | 41.3 | 40.2 | 40.7 | ||
| High | 60.1 | 55.7 | 56.8 | 56.3 | ||
| Comorbid conditions, % | ||||||
| Diabetes mellitus | 12.4 | 9.9 | 0.08 | 10.5 | 10.3 | 0.01 |
| Hypertension | 26.5 | 22.1 | 0.10 | 23.1 | 22.7 | 0.01 |
| Hyperlipidemia | 14.2 | 11.6 | 0.08 | 12.2 | 11.9 | 0.01 |
| Myocardial infarction | 0.5 | 0.2 | 0.03 | 0.3 | 0.3 | 0.00 |
| Stroke | 4.6 | 2.2 | 0.13 | 2.6 | 2.5 | 0.00 |
| Angina | 2.3 | 1.8 | 0.04 | 2.0 | 1.8 | 0.01 |
| Upper gastrointestinal tract disease | 18.5 | 13.3 | 0.14 | 14.6 | 14.0 | 0.02 |
| Fractures | 6.4 | 3.0 | 0.16 | 3.8 | 3.5 | 0.02 |
| Osteoporosis | 8.5 | 4.9 | 0.15 | 5.6 | 5.3 | 0.01 |
| Liver disease | 13.9 | 10.6 | 0.10 | 11.2 | 11.0 | 0.01 |
| Chronic back pain | 36.8 | 29.6 | 0.15 | 31.0 | 30.5 | 0.01 |
| Gout | 8.3 | 11.4 | 0.10 | 11.1 | 11.0 | 0.00 |
| Heart failure | 2.4 | 1.5 | 0.07 | 1.7 | 1.6 | 0.01 |
| Osteoarthritis | 31.1 | 24.4 | 0.15 | 25.2 | 25.2 | 0.00 |
| Chronic renal disease | 2.6 | 1.0 | 0.12 | 1.3 | 1.2 | 0.01 |
| Comedications, % | ||||||
| Proton pump inhibitor | 8.1 | 4.2 | 0.16 | 4.9 | 4.7 | 0.01 |
| Antithrombotic therapy | 8.6 | 5.5 | 0.12 | 6.2 | 5.9 | 0.02 |
| β Blockers | 17.2 | 13.6 | 0.10 | 14.4 | 14.1 | 0.01 |
| ACEI | 6.5 | 5.5 | 0.03 | 5.8 | 5.6 | 0.01 |
| ARB | 12.1 | 7.9 | 0.14 | 9.0 | 8.5 | 0.02 |
| Oral steroid | 37.9 | 41.0 | 0.06 | 41.0 | 40.6 | 0.01 |
| DMARD regimens, %b | 0.20 | 0.01 | ||||
| No use | 69.3 | 65.6 | 65.7 | 66.0 | ||
| Methotrexate combination | 7.1 | 5.9 | 6.0 | 6.0 | ||
| Other methotrexate‐free combination | 3.9 | 3.6 | 3.6 | 3.6 | ||
| Methotrexate only | 1.4 | 2.3 | 2.2 | 2.2 | ||
| Hydroxychloroquine only | 12.5 | 15.6 | 15.6 | 15.3 | ||
| Sulfasalazine only | 3.3 | 6.0 | 5.5 | 5.6 | ||
| Other DMARD monotherapy | 0.7 | 0.4 | 0.5 | 0.5 | ||
| bDMARD regimens | 1.8 | 0.7 | 0.8 | 0.8 | ||
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; bDMARD, biologic‐containing DMARD; DMARD, disease‐modifying antirheumatic drug; IPTW, inverse probability of treatment weight; PS, propensity score; and SMD, standardized mean difference.
SMD ≥0.1 represented significant differences.
Although using any DMARDs was one of the inclusion criteria, some patients may start taking NSAIDs before DMARDs or stop DMARDs during the follow‐up. Methotrexate combination indicates biologic‐free methotrexate combination regimens; other methotrexate‐free combination, biologic‐ and methotrexate‐free combination regimens; and bDMARD regimens, any monotherapy or combination regimen containing biologics.
Incidence and Risk of Composite Cardiovascular Events in Topical and Oral Nonselective NSAID Treatment Episodes
The crude cardiovascular event rates were 1.83 per 100 person‐years in the topical nonselective NSAID group and 2.14 per 100 person‐years in the oral nonselective NSAID group (Table 2). The breakdown of numbers of composite cardiovascular events was presented in Table S3. The Kaplan‐Meier survival curve demonstrated that the topical nonselective NSAID group had a trend for higher event‐free survival during the follow‐up than the oral nonselective NSAID group (P=0.25 for log‐rank test) (Figure 2). Both of the IPTW weighted and multivariable Cox regression models found topical NSAID users had significantly lower risk of composite cardiovascular events compared with oral NSAID users: hazard ratio (HR; IPTW weighted model), 0.64 (95% confidence interval [CI], 0.43–0.95); and HR (multivariable model), 0.54 (95% CI, 0.37–0.77) (Table 2).
Table 2.
Incidence and Risk of Composite Cardiovascular Events in Topical and Oral Nonselective NSAID Users
| Type of NSAID Use | Follow‐Up, Person‐Years | No. of Events | Crude Incidence per 100 Person‐Years | IPTW Weighted Cox Modela, b | Multivariate Cox Modela, c |
|---|---|---|---|---|---|
| Topical (N=10 758) | 1854.6 | 34 | 1.83 | 0.64 (0.43–0.95) | 0.54 (0.37–0.77) |
| Oral (N=78 056) | 20 205.3 | 433 | 2.14 | REF | REF |
IPTW indicates inverse probability of treatment weight; and REF, reference.
Data are given as hazard ratio (95% confidence interval).
IPTW was derived on the basis of the propensity score, which was calculated from the multivariable logistic regression model, conditional on all variables in Table 1.
Adjusted for all covariates listed in the Methods.
Figure 2.
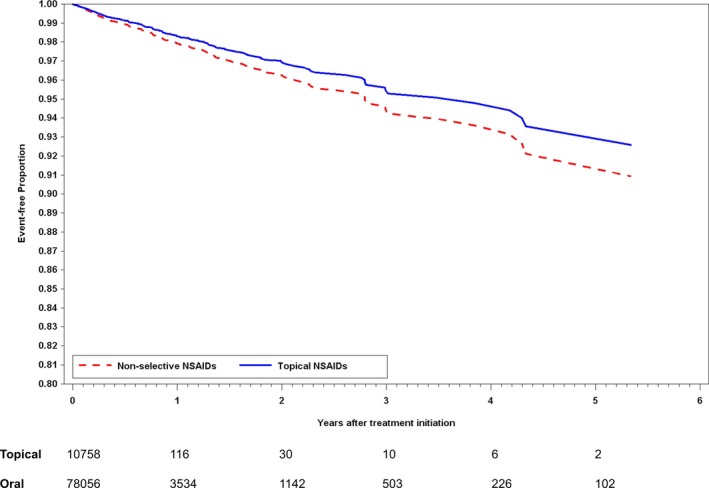
Kaplan‐Meier estimates for composite cardiovascular events (P=0.2453 for log‐rank test).
Results of sensitivity analyses are shown in Figure 3. We first matched 6722 and 47 024 topical and oral nonselective NSAID treatment episodes by PS in a 1:7 ratio and found consistent results: HR, 0.52 (95% CI, 0.27–0.98). After excluding episodes started before DMARD initiation, there were 9366 topical and 61 762 oral nonselective NSAID treatment episodes identified for analysis. Similar results were found in this sensitivity analysis (HR, 0.67; 95% CI, 0.44–1.02). Consistent direction of HRs was found when we extended the grace period to 60 days and in subgroup analyses.
Figure 3.
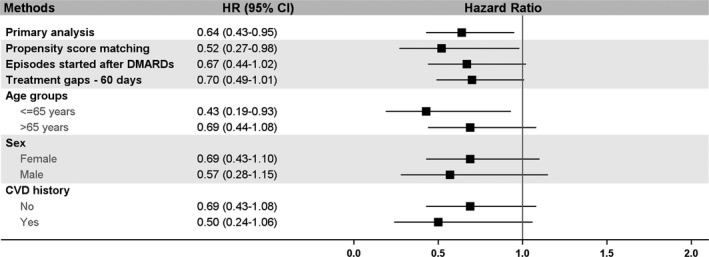
Results of sensitivity analyses. Hazard ratios (HRs) were generated from inverse probability of treatment weight weighted Cox models. No cardiovascular disease (CVD) history: no myocardial infarction, stroke, angina, or heart failure during the baseline period. Episodes started after disease‐modifying antirheumatic drugs (DMARDs): excluding NSAID episodes that started before DMARD initiation. CI indicates confidence interval.
Discussion
Theoretically, topical NSAIDs may have lower systemic adverse events than oral NSAIDs because of pharmacokinetic and pharmacodynamic actions. In this retrospective cohort of Taiwanese patients with incident RA, aged >18 years, we found the incidence and risk of composite cardiovascular events were significantly lower in topical nonselective NSAID users than in those receiving oral nonselective NSAIDs in the primary analysis. The direction of results was consistent across sensitivity analyses. However, these analyses were not statistically significant because of limited power.
To our knowledge, the current study was the first head‐to‐head comparison of the cardiovascular outcomes in patients with RA who were receiving topical or oral nonselective NSAIDs. There are several preclinical and clinical studies suggesting that topical NSAIDs have comparable efficacy in symptom relief in osteoarthritis and inflammatory arthritis10, 18, 19, 20 and fewer gastrointestinal tract and hepatic adverse effects with their oral forms in patients with arthritis.10, 21 However, these comparisons of safety outcomes between topical and oral NSAIDs were often limited to small sample size with <12 weeks of follow‐up, and there were few cardiovascular outcome studies for NSAIDs in patients with RA. In 2011, Roth et al evaluated the safety outcomes in topical and oral diclofenac users by pooling data from two 12‐week, double‐blind, randomized controlled trials.13, 21, 22 There were 927 patients, aged between 40 and 85 years (n=465 for topical diclofenac, n=462 for oral diclofenac), from Canada and the United States with osteoarthritis included in this post hoc analysis. They found the topical diclofenac group had lower rates for any vascular or cardiac‐related adverse event (1.5% versus 3.5%; P=0.055) and cardiac disorder (0.2% versus 1.3%, including angina, acute myocardial infarction, arrhythmia, coronary artery disease, and palpitations) in comparison to the oral diclofenac group during the 12 weeks of follow‐up.13 Our results extend the findings of Roth and Fuller from the previous post hoc analysis13 in a large Taiwanese RA cohort under real‐world practices. Compared with the post hoc analysis,13 our cohort was slightly younger and included more cardiovascular diseases (heart failure, stroke, and revascularization) as study outcomes. Our results also supported that topical nonselective NSAID users had a lower cardiovascular event rate (crude rates, 1.8 per 100 person‐years for topical nonselective NSAIDs and 2.1 per 100 person‐years for oral nonselective NSAIDs), and there was a 36% lower risk for the composite cardiovascular events in topical nonselective NSAID users than in oral nonselective NSAID users (PS adjusted HR, 0.64; 95% CI, 0.43–0.95).
We also found topical nonselective NSAID users may have a lower risk for cardiovascular events in the subgroup of patients with preexisting cardiovascular diseases within 6 months before each treatment episode, defined by having myocardial infarction, stroke, angina, or heart failure. On the basis of results from a recent meta‐analysis,3 the US Food and Drug Administration strengthened the warning that oral nonselective NSAIDs were associated with a higher incidence of excess serious cardiovascular events or death in patients with preexisting cardiovascular disease, recent MI, and severe heart failure.14 Consideration of using topical nonselective NSAIDs in patients with cardiovascular diseases may have 2 advantages. First, one of the known mechanisms for increasing cardiovascular risk among COX‐2 inhibitors and oral nonselective NSAIDs is inhibition of the COX‐2 enzyme.23 Use of topical nonselective NSAIDs in patients with known cardiovascular diseases may provide benefit from less systemic inhibition of COX‐2 enzyme and thus lower cardiovascular risk. This has been evidenced in a recent randomized crossover pharmacodynamic study; it found that topical diclofenac only inhibited approximately half of COX‐2 as oral diclofenac did in the systemic form.9 Second, the potential of drug‐drug interaction between aspirin and oral nonselective NSAIDs might be an issue in patients with known cardiovascular diseases because aspirin is the anchor drug for secondary prevention of cardiac thrombotic events. In 2016, Rowcliffe et al found oral diclofenac did inhibit the beneficial action of aspirin by reversibly binding to the COX‐1 enzyme, resulting in a significant reduction in platelet inhibition, whereas no interaction was observed between topical diclofenac and aspirin.24 This drug‐drug interaction could contribute to aspirin therapy failure in patients who underwent secondary prevention for myocardial infarction or strokes.25 Currently, the topical NSAIDs have the same warnings and precautions for cardiovascular events with their oral form in their package insert in the United States.26 Our findings suggest a potential beneficial role of topical nonselective NSAIDs in patients with preexisting cardiovascular diseases, which warrant further confirmation in future larger studies.
There were several strengths in our study. First, we chose the population with RA, who commonly use oral or topical NSAID treatments, providing us a large cohort to examine the association. Second, we included many potential confounders from our claims database and used several explicit pharmacoepidemiological methods, including PS weighting and matching; distributions of covariates showed excellent balance across groups after weighting. Several limitations should be addressed. First, although we adopted a validated algorithm to identify patients with incident RA by acquiring all patients who received any DMARD within the first year of diagnosis, patients may start NSAID therapy first before the DMARD initiation in typical clinical practices. We further examined the study outcomes in treatment episodes exclusively started after DMARD initiation and found consistent rates (1.90 in topical versus 2.09 in oral NSAIDs/100 person‐years) and HRs. Therefore, the effects of timing of starting DMARDs seem to be minimal in our study. Second, the number of composite cardiovascular events (N=34) was limited in the topical nonselective NSAID groups, which prohibited us to compare the risk in individual cardiovascular outcomes. Third, the window for ascertainment of cardiovascular disease history and other comorbidities in the current study was 6 months. This is a tradeoff to get an extended baseline period or a larger sample size for treatment episodes. It is possible that we underestimated the prevalence of cardiovascular disease history and other comorbidities, especially MI and stroke, leading to diminishing the effectiveness of propensity scoring to control for potential confounding. However, the rates observed in our study were within the range of published Asian RA literature.27, 28 A recently published study also found that using all available data to capture baseline covariates may have higher sensitivity, but did not meaningfully change the overall treatment effect estimates.29 Third, the mean follow‐up duration in the topical nonselective NSAID group was ≈2 months, reflecting the short‐term treatment pattern of topical NSAIDs in the real‐life setting. Although we had longer follow‐up and events when we extended the treatment gap to 60 days, the effects attenuated, possibly because of misclassification bias. The exposure scenario under more extended treatment gaps was usually closer to the intent‐to‐treat design, which aims to provide more conservative results but also may underestimate the treatment adverse effects when patients did not fully adhere to the regimens.30 Nevertheless, on the basis of the reviews and latest statement from the US Food and Drug Administration, the risk of cardiovascular events can occur as early as the first weeks of using an NSAID.14 Our study could reflect the early cardiovascular risk information in patients newly taking either topical or oral NSAIDs. Fourth, we did not have information about how patients applied their topical nonselective NSAIDs to their body in our claims database. Variations might exist between patients in the amount of actual doses entering systematic circulation. We also did not have RA disease activity or inflammatory marker (eg, C‐reactive protein or erythrocyte sedimentation rate) information in our claims data. It is possible that physicians prefer oral NSAIDs over topical NSAIDs if patients have higher disease activity or polyarticular flares. We, therefore, established a DMARD regimen variable, which included 7 classes of regimens and included previous diagnoses of osteoarthritis and low back pain as surrogates for RA disease activity in the PS adjustment. In addition, the DMARD regimens noted in Table 1 are similar in distribution across the oral and topical NSAID regimens. We also did not have smoking information in our claims database. However, our cohort was predominantly Taiwanese women (≈80%), and it was reported that the smoking prevalence was only 4% to 5% during the study period in the governmental survey.31 Furthermore, the results were consistent in female and male subgroups. Finally, our study focused on patients with RA, and results might not be generalized to other populations, such as patients with osteoarthritis.
In conclusion, our hypothesis‐driven study found topical nonselective NSAIDs were associated with a lower risk for composite cardiovascular events compared with oral nonselective NSAIDs in Taiwanese patients with RA. Currently, the US Food and Drug Administration strengthened the label warnings for the risk of cardiovascular events with all NSAID use, regardless of the routes of administration and preexisting cardiovascular diseases.14 Together with the results from a previous pharmacodynamic study,9, 24 our results suggested that topical nonselective NSAIDs may be a safer alternative for relieving muscle‐skeletal pain in patients with cardiovascular diseases, including those receiving aspirin concurrently. Future trials or observational studies with more events and longer‐term follow‐up will help us understand more about the risk difference between topical and oral NSAIDs in individual cardiovascular outcomes. Results in patients with osteoarthritis and head‐to‐head comparisons of different formulations within topical NSAIDs (eg, gel, ointment, and patch) are also needed.
Disclosures
Solomon receives salary support from grants to Brigham and Women's Hospital from Pfizer, Eli Lilly, Amgen, Bristol Myers Squibb, and Genentech. He has also served without pay on the Executive Committee of a large NSAID trial funded by Pfizer. Furthermore, he receives royalties for chapters in UpToDate about NSAIDs. The remaining authors have no disclosures to report.
Supporting information
Table S1. Operational Definitions
Table S2. Characteristics of Treatment Episodes in the Original and IPTW Weighted Cohorts Using Topical or Oral Non‐Selective NSAIDs
Table S3. The Breakdown of Composite Outcomes and Death Events
(J Am Heart Assoc. 2017;6:e006874 DOI: 10.1161/JAHA.117.006874.)29079568
References
- 1. Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, Canning C, Schneeweiss S. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis. 2006;65:1608–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, Stampfer MJ, Curhan GC. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. [DOI] [PubMed] [Google Scholar]
- 3. Coxib Traditional NSAID Trialists’ Collaboration , Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA, Goss P, Halls H, Hawk E, Hawkey C, Hennekens C, Hochberg M, Holland LE, Kearney PM, Laine L, Lanas A, Lance P, Laupacis A, Oates J, Patrono C, Schnitzer TJ, Solomon S, Tugwell P, Wilson K, Wittes J, Baigent C. Vascular and upper gastrointestinal effects of non‐steroidal anti‐inflammatory drugs: meta‐analyses of individual participant data from randomised trials. Lancet. 2013;382:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, Egger M, Juni P. Cardiovascular safety of non‐steroidal anti‐inflammatory drugs: network meta‐analysis. BMJ. 2011;342:c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salvo F, Fourrier‐Reglat A, Bazin F, Robinson P, Riera‐Guardia N, Haag M, Caputi AP, Moore N, Sturkenboom MC, Pariente A; Investigators of Safety of Non‐Steroidal Anti‐Inflammatory Drugs: SOS Project . Cardiovascular and gastrointestinal safety of NSAIDs: a systematic review of meta‐analyses of randomized clinical trials. Clin Pharmacol Ther. 2011;89:855–866. [DOI] [PubMed] [Google Scholar]
- 6. van Walsem A, Pandhi S, Nixon RM, Guyot P, Karabis A, Moore RA. Relative benefit‐risk comparing diclofenac to other traditional non‐steroidal anti‐inflammatory drugs and cyclooxygenase‐2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: a network meta‐analysis. Arthritis Res Ther. 2015;17:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170:1968–1976. [DOI] [PubMed] [Google Scholar]
- 8. Goodson NJ, Brookhart AM, Symmons DP, Silman AJ, Solomon DH. Non‐steroidal anti‐inflammatory drug use does not appear to be associated with increased cardiovascular mortality in patients with inflammatory polyarthritis: results from a primary care based inception cohort of patients. Ann Rheum Dis. 2009;68:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50:50–61. [DOI] [PubMed] [Google Scholar]
- 10. Derry S, Moore RA, Gaskell H, McIntyre M, Wiffen PJ. Topical NSAIDs for acute musculoskeletal pain in adults. Cochrane Database Syst Rev. 2015;6:CD007402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baraf HS, Gold MS, Petruschke RA, Wieman MS. Tolerability of topical diclofenac sodium 1% gel for osteoarthritis in seniors and patients with comorbidities. Am J Geriatr Pharmacother. 2012;10:47–60. [DOI] [PubMed] [Google Scholar]
- 12. Taylor RS, Fotopoulos G, Maibach H. Safety profile of topical diclofenac: a meta‐analysis of blinded, randomized, controlled trials in musculoskeletal conditions. Curr Med Res Opin. 2011;27:605–622. [DOI] [PubMed] [Google Scholar]
- 13. Roth SH, Fuller P. Diclofenac topical solution compared with oral diclofenac: a pooled safety analysis. J Pain Res. 2011;4:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. FDA strengthens warning that non‐aspirin nonsteroidal anti‐inflammatory drugs (NSAIDs) can cause heart attacks or strokes. 2015. http://www.Fda.Gov/drugs/drugsafety/ucm451800.Htm. Accessed June 28, 2016.
- 15. Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, Solomon DH. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV‐positive men. Epidemiology. 2000;11:561–570. [DOI] [PubMed] [Google Scholar]
- 17. Calabrese LH, Calabrese C, Kirchner E. The 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis should include new standards for hepatitis B screening: comment on the article by Singh et al. Arthritis Care Res (Hoboken). 2016;68:723–724. [DOI] [PubMed] [Google Scholar]
- 18. Mason L, Moore RA, Edwards JE, Derry S, McQuay HJ. Topical NSAIDs for chronic musculoskeletal pain: systematic review and meta‐analysis. BMC Musculoskelet Disord. 2004;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heyneman CA, Lawless‐Liday C, Wall GC. Oral versus topical NSAIDs in rheumatic diseases: a comparison. Drugs. 2000;60:555–574. [DOI] [PubMed] [Google Scholar]
- 20. Singh G. Recent considerations in nonsteroidal anti‐inflammatory drug gastropathy. Am J Med. 1998;105:31S–38S. [DOI] [PubMed] [Google Scholar]
- 21. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002–2012. [PubMed] [Google Scholar]
- 22. Simon LS, Grierson LM, Naseer Z, Bookman AA, Zev Shainhouse J. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis. Pain. 2009;143:238–245. [DOI] [PubMed] [Google Scholar]
- 23. Ong CK, Lirk P, Tan CH, Seymour RA. An evidence‐based update on nonsteroidal anti‐inflammatory drugs. Clin Med Res. 2007;5:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rowcliffe M, Nezami B, Westphal ES, Rainka M, Janda M, Bates V, Gengo F. Topical diclofenac does not affect the antiplatelet properties of aspirin as compared to the intermediate effects of oral diclofenac: a prospective, randomized, complete crossover study. J Clin Pharmacol. 2016;56:422–428. [DOI] [PubMed] [Google Scholar]
- 25. Saxena A, Balaramnavar VM, Hohlfeld T, Saxena AK. Drug/drug interaction of common NSAIDs with antiplatelet effect of aspirin in human platelets. Eur J Pharmacol. 2013;721:215–224. [DOI] [PubMed] [Google Scholar]
- 26. Tsutsumi Y, Ogasawara R, Kamihara Y, Ito S, Yamamoto Y, Tanaka J, Asaka M, Imamura M. Rituximab administration and reactivation of HBV. Hepat Res Treat. 2010;2010:182067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chung WS, Lin CL, Peng CL, Chen YF, Lu CC, Sung FC, Kao CH. Rheumatoid arthritis and risk of acute myocardial infarction: a nationwide retrospective cohort study. Int J Cardiol. 2013;168:4750–4754. [DOI] [PubMed] [Google Scholar]
- 28. Jeong H, Baek SY, Kim SW, Eun YH, Kim IY, Kim H, Lee J, Koh EM, Cha HS. Comorbidities of rheumatoid arthritis: results from the Korean National Health and Nutrition Examination Survey. PLoS One. 2017;12:e0176260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakasian SS, Rassen JA, Franklin JM. Effects of expanding the look‐back period to all available data in the assessment of covariates. Pharmacoepidemiol Drug Saf. 2017;26:890–899. [DOI] [PubMed] [Google Scholar]
- 30. Hernan MA, Hernandez‐Diaz S. Beyond the intention‐to‐treat in comparative effectiveness research. Clin Trials. 2012;9:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adult smoking behavior surveillance system, health promotion administration, ministry of health and welfare, Taiwan. http://tobacco.Hpa.Gov.Tw/show.Aspx?Menuid=581. Accessed August 10, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Operational Definitions
Table S2. Characteristics of Treatment Episodes in the Original and IPTW Weighted Cohorts Using Topical or Oral Non‐Selective NSAIDs
Table S3. The Breakdown of Composite Outcomes and Death Events


