Figure 8.
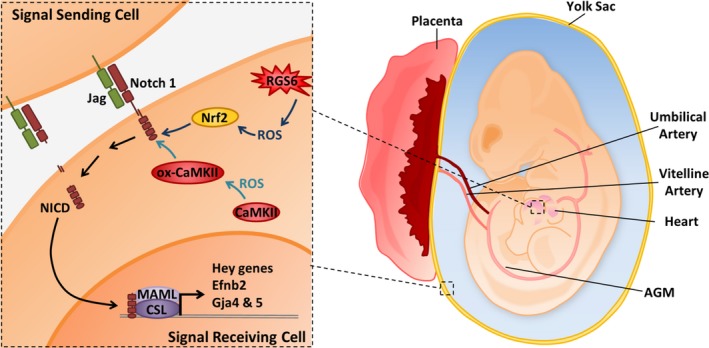
Schematic illustration of Regulator of G protein signaling 6 (RGS6)– and/or Ca2+/calmodulin‐dependent protein kinase II (CaMKII)–dependent activation of Notch signaling in embryonic heart and vasculature. In this article we provide evidence that RGS6 and oxidated CaMKII (ox‐CaMKII) function in parallel pathways as critical upstream modulators of Notch signaling. RGS6‐mediated reactive oxygen species (ROS) generation modulates Nrf2 levels while RGS6‐independent ROS oxidizes CaMKII to control Notch signaling for proper cardiovascular development. We hypothesize that together, RGS6 and ox‐CaMKII in signal‐receiving cells (receptor‐ expressing cells), which promote Notch1 receptor and ligand (Jagged [Jag]) interaction and culminate in the proteolytic cleavage of Notch and the release of the Notch intracellular domain (NICD) into the cytoplasm, NICD translocation into the nucleus, and NICD‐mediated gene transcription upon association with other transcriptional regulators CBF1/Su(H)/Lag‐1, CSL, and mastermind‐like (MAML). However, it is possible that RGS6 and ox‐CaMKII are also present in signal‐sending cells (ligand‐expressing cells) and work in a similar manner. In addition, the parallel pathways utilized by RGS6 and ox‐CaMKII (including their respective ROS; RGS6‐dependent ROS [moderate‐high level] and non‐RGS6‐ROS [basal‐low level]) are denoted using different shades of blue to emphasize their separate and independent functions. AGM indicates aorta‐gonad‐mesonephros (contains the dorsal aorta); Efnb2, Ephrin B2.
