Abstract
Background
Previous studies observe associations between lifetime parity and cardiovascular disease, but relatively fewer investigate age at first childbirth (AFB). Herein, we examine the association of AFB with a summary cardiovascular risk measure (Framingham Risk Score [FRS]).
Methods and Results
As part of the IMIAS (International Mobility in Aging Study), data were collected in 2012 among 1047 women, aged 65 to 74 years, from Canada, Albania, Colombia, and Brazil. FRSs were calculated to describe cardiovascular risk profiles, and linear regression analyses were performed, adjusting for early life and socioeconomic variables. Women with an AFB of <20 years were compared with women with an AFB of 20 to 24, 25 to 29, and ≥30 years, as well as nulliparous women. We also compared FRS between combinations of AFB and parity categories: nulliparous women, parity 1 to 3 combined with AFB <20 years, parity ≥4 with AFB <20 years, parity 1 to 3 with AFB ≥20 years, and parity ≥4 with AFB ≥20 years. Women with an AFB of <20 years had a higher mean FRS compared with all other AFB groups. Compared with the lowest AFB risk group (25–29 years), women with an AFB of <20 years had a 5.8‐point higher mean FRS (95% confidence interval, 3.4–8.3 points). Nulliparous women presented the lowest mean FRS in all analyses. The analysis comparing combinations of AFB and parity categories showed no meaningful differences in FRS between women who had 1 to 3 childbirths and those who had ≥4 childbirths within the stratum of AFB <20 years, and in the stratum of AFB ≥20 years.
Conclusions
Our analyses suggest that nulliparity and AFB, rather than increasing parity, drive the association with cardiovascular disease risk.
Keywords: age at first birth, cardiovascular disease risk factors, epidemiology, Framingham Risk Scores, global health
Subject Categories: Aging, Cardiovascular Disease, Pregnancy, Risk Factors, Women
Clinical Perspective
What Is New?
To the best of our knowledge, this is the first study to demonstrate, in postmenopausal women from multiple global settings, that adolescent childbirth is related to greater overall cardiovascular risk, as measured by the Framingham Risk Score, compared with women who gave birth at later ages, and compared with nulliparous women.
What Are the Clinical Implications?
Adolescent childbirth may serve as a cardiovascular disease risk marker; women who were adolescent mothers may benefit from earlier and increased cardiovascular screening to reduce the incidence of cardiovascular events.
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide.1 Women's reproductive health is increasingly recognized as an important contributing factor to CVD risk.2, 3, 4, 5, 6 Previous studies document associations of greater lifetime parity,2, 7 early age at menarche,3, 8 and early age at menopause4 with CVD; however, findings are not always consistent.9, 10 Age at first childbirth (AFB) is much less studied. In this article, we focus on 2 aspects of women's reproductive health (AFB and parity) as determinants of CVD risk in an international sample of postmenopausal women.
Numerous studies observe associations between an early AFB, especially adolescent childbirth, and all‐cause mortality11, 12, 13, 14 and a few observe associations between AFB and CVD risk factors, CVD events, and CVD mortality.6, 15, 16, 17, 18 Parity has been examined more thoroughly than AFB,2, 4, 19, 20, 21, 22 including as part of 2 systematic reviews on women's cardiovascular health.4, 19 In those studies reporting statistically significant associations, typically nulliparous and multiparous women are at greater risk of CVD.4, 19, 21 Yet, AFB may confound observed associations between parity and CVD, because AFB is closely linked with parity.18 Women with early AFB tend to have more children.23, 24 For example, in 1 study of middle‐aged women from Brazil, those who gave birth as adolescents were twice as likely to have ≥3 children across their lifetimes compared with women whose first childbirth was during adulthood.23
AFB may influence CVD through pathways that range from the purely physiological to the social and behavioral.15, 17 On one end of the spectrum, pregnancy‐related physiological changes may differ in adolescent compared with adult women.25, 26, 27, 28, 29 For example, adolescent pregnant women may be exposed to physiological changes accompanying pregnancy, such as insulin resistance, that irreversibly influence cardiovascular health earlier in life. These changes contribute to longer exposure durations and, thus, greater overall CVD risk compared with women who have children later in life.15, 30 In addition, well‐conducted prospective studies document greater weight gain and retention among pregnant adolescents compared with adult pregnant women, likely contributing to later‐life obesity and cardiometabolic diseases.25, 26 On the other end of the spectrum, associations between AFB and CVD may be entirely social. The age at which a woman gives birth likely has long‐lasting consequences on educational attainment, career opportunities, family relations, and family support.31, 32, 33 The accumulation of adversities during a woman's life course potentially accelerates aging and increases metabolic and cardiovascular risk through mechanisms such as multisystem dysregulation (eg, greater allostatic load).34, 35
By using data from the IMIAS (International Mobility in Aging Study), we investigate the relationship between self‐reported AFB, lifetime parity, and the Framingham Risk Score (FRS), a summary measure of CVD risk. Because of previous research on lifetime parity and CVD, we aim to disassociate the effects of AFB from parity. This cohort of community‐dwelling older adults is unique in that it allows for examination of the hypothesized association (ie, early childbirth is associated with greater CVD risk) in 5 diverse settings with widely differing levels of exposures and outcome.
Methods
The IMIAS
Data were collected as part of the IMIAS, a prospective cohort study of community‐dwelling older adults, conducted at 5 sites: Tirana (Albania), Natal (Brazil), Manizales (Colombia), Kingston (Ontario, Canada), and Saint‐Hyacinthe (Quebec, Canada). Albania, Colombia, and Brazil are considered middle‐income countries and, on average, have lower educational attainment, lower human development indexes, and higher income and gender inequalities compared with Canada.36
The IMIAS cohort was established in 2012 and contains 2002 community‐dwelling older adults, aged 65 to 74 years at the time of recruitment. Approximately 200 women were enrolled at each of the 5 sites. Participants with ≥4 errors on the orientation scale of the Leganes Cognitive Test, a screening tool for dementia in populations with low levels of education,37, 38 were excluded (0 in Kingston, 1 in Saint‐Hyacinthe and Tirana each, 2 in Manizales, and 5 in Natal). These participants were excluded because they were likely unable to complete the study protocols. Of the 2002 participants recruited to IMIAS, 1047 are women.
Recruitment and Study Procedures
In Manizales, ≈82% of the older adults are covered in the public healthcare network, and the populations of Brazil, Albania, and Canada have universal healthcare coverage, facilitating affordable access to healthcare providers for most older adults. Because high proportions of older adults in these contexts are registered in the public health system, we recruited through primary care centers. In Tirana, Natal, and Manizales, participants were randomly selected from the population registered at neighborhood health centers and invited to participate in the study. Response rates were >90% in Tirana, Manizales, and Natal. Ethics' committees at the 2 Canadian universities did not allow direct contact with potential participants. Canadian participants were, thus, invited by a letter from their healthcare provider, encouraging them to contact our field coordinator if they were interested in participating in the study. In Saint‐Hyacinthe and Kingston, 30% of the invited subjects contacted the field coordinator. Of them, 95% participated in the study. Comparisons of the population sampled in Canada with census data suggest that participants recruited in Saint‐Hyacinthe are representative of the population of that city, whereas those in Kingston are slightly better educated than the general population of older adults of that city.
Unless otherwise requested, interviews were conducted at the participants' homes. Study questionnaires and supporting materials were available in all local languages and administered by interviewers who were trained according to the same protocol and standards. Anthropometric measures and blood samples were also obtained at baseline. Blood samples were analyzed for several cardiovascular and metabolic biomarkers. Analyses were conducted at the Medical School University Hospital at Tirana (Tirana, Albania); Multilab Laboratory and Alvaro Laboratorios (Natal, Brazil); Caldas University Hospital (Manizales, Colombia); Kingston General Hospital (Kingston, Ontario, Canada); and the Honoré Mercier Hospital (Saint‐Hyacinthe, Quebec, Canada). More details on the study sites, populations, and recruitment can be found in the study by Zunzunegui et al.39 Of the 1047 women who participated in the IMIAS baseline data collection, cardiometabolic data from blood samples were available for 908 (87%). Complete data on all covariates included in this study (see measures below) were available for 892 women (85%), or 98% of those with FRS data.
Human Subjects
Institutional review for this project was obtained from the relevant organizations at each site: the Institute of Public Health in Albania, the Federal University of Rio Grande do Norte in Brazil, the University of Caldas in Colombia, the University of Montreal Hospital Research Centre, and Queen's University in Canada. Written informed consent was obtained from all participants.
Primary Outcome Measure
FRSs are based on the Framingham Heart Study, a long‐term cardiovascular cohort study, ongoing in Framingham, MA.40 Several FRSs have been developed to estimate the CVD, coronary heart disease, and stroke risk of an individual. We use 10‐year laboratory‐based CVD risk equations based on sex, age, systolic blood pressure, treatment for hypertension, total and high‐density lipoprotein cholesterol, smoking, and diabetes mellitus status.41
Blood pressure was measured 3 times after at least 5 minutes of rest using a validated automated blood pressure device. The mean value of the second and third systolic blood pressure measurements was used to inform the Framingham equation. As part of the survey, participants were requested to show the interviewer all containers of medication that they used; these were recorded. A participant was considered to be taking antihypertensive medication when either antihypertensive medication was presented or the participant self‐reported using antihypertensive medication. Cholesterol and glycosylated hemoglobin measures were obtained through a blood sample collected by a trained phlebotomist. Participants were considered to have diabetes mellitus based on a high glycosylated hemoglobin level (≥6.5%),42 antidiabetic medication, and/or self‐reported diabetes mellitus. The latter was determined by an affirmative response to the question “Has a physician or nurse ever told you that you have diabetes mellitus (ie, high blood glucose levels)?” Smoking and age were self‐reported.
Exposure Variables
AFB and parity were self‐reported. We operationalized AFB into 5 categories: <20 years, 20 to 24 years, 25 to 29 years, ≥30 years, and nulliparous women. Most studies examining AFB and CVD events have defined their youngest age group as <20 years.6, 18 Because we hypothesize that younger AFB is associated with greater CVD risk, we treated AFB <20 years as the reference group. To dissociate between AFB and parity when examining the association with FRS, we additionally conducted analyses in which we compared FRS according to combinations of AFB and parity categories. We categorized women into 5 groups: nulliparous women, parity 1 to 3 combined with AFB <20 years, parity ≥4 combined with AFB <20 years, parity 1 to 3 combined with AFB ≥20 years, and parity ≥4 combined with AFB ≥20 years. These parity categories were selected on the basis of previous work suggesting that parity is associated with CVD in a J‐shaped manner, with the lowest risk observed among women with 2 births.21
Covariates
Age and study site
The trained interviewer recorded these data.
Childhood social and economic adversity
On the basis of a previous validation study with the IMIAS data,43 a participant was considered to have experienced childhood social adversity if she reported parental alcohol or drug abuse, witnessing physical violence in the family, and/or physical abuse during childhood. A participant was considered to have experienced childhood economic adversity if she reported poor economic status, hunger, or unwanted parental unemployment. Childhood adversities were self‐reported and pertained to the first 15 years of life.43
Education and income
Educational level was categorized into tertiles (low, medium, and high) of self‐reported years of education. Because of the large difference in years of education between the research sites, these tertiles were determined site specifically as follows: Kingston, <15, 15 to 17, and ≥18 years; Saint‐Hyacinthe, <11, 11 to 12, and ≥13 years; Tirana, <8, 8 to 12, and ≥13 years; and Latin American sites, <4, 4 to 5, and ≥6 years. Participant income level was site specific and classified into 3 categories (poor, middle, and middle high/high), as follows: Canadian sites, annual income of <20 000, 20 000 to 40 000, >40 000 CAD; Latin American sites, monthly income of <1 minimum salary, 1 minimum salary, and ≥2 minimum salaries; and Albania, annual income of <1000, 1000 to 2000, and ≥2000 USD.
Statistical Analyses and Conceptual Framework
We explored the population characteristics of the sample with descriptive statistics. In bivariate analyses, we used 2‐sample independent t tests for dichotomous variables and ANOVA for polytomous variables to examine associations between population characteristics and FRS.
Figure 1 depicts our conceptual framework with relevant citations supporting each arrow in the model.6, 15, 16, 17, 44, 45, 46, 47, 48, 49, 50, 51, 52 Directed acyclic graphs were used to establish the minimum confounders required for the multivariate analyses. With linear regression models, we examined the covariate‐adjusted associations between AFB, parity, and FRS. The models were built in a step‐wise manner based on our conceptual framework. Accordingly, participant age and study site were considered as confounders in all models. Childhood social and economic adversities were included as dichotomous variables in the second model, whereas educational level and income were added to the third model. We consider the covariates added in the first 2 models as likely confounders. The third model is exploratory. The covariates added in the last model may confound the association between AFB and CVD risk, but may also be on the pathway between AFB and CVD risk, in which case their inclusion in the model would entail overadjustment. For example, an environment with lower educational opportunities may predispose women to have children at a young age. At the same time, having a child at a young age may also influence future educational and professional opportunities. In the analyses of the combined AFB and parity categories, we statistically adjust only for the likely confounders (age, study site, and childhood adversities) because the third model is more exploratory.
Figure 1.
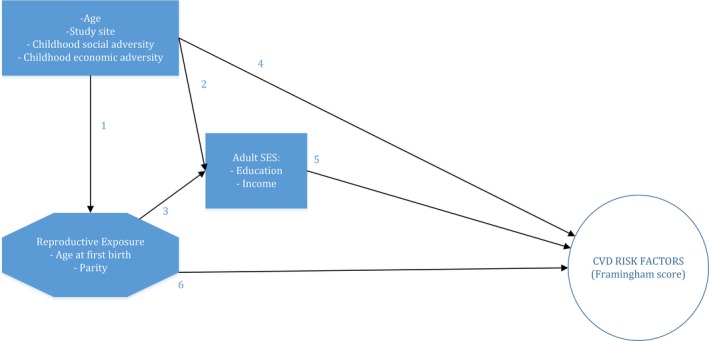
Conceptual framework. The references corresponding to each arrow (a previously published association) are indicated as follows: (1) references 45, 46; (2) reference 45; (3) reference 45; (4) references 47, 48, 49, 50; (5) references 44, 51, 52; and (6) references 6, 15, 16, 17. AFB indicates age at first childbirth; CVD, cardiovascular disease; FRS, Framingham Risk Score; and SES, socioeconomic status.
Finally, sensitivity analyses were conducted to assess the robustness of our findings, because not all study participants agreed to have laboratory measures taken. For 139 women, we could not calculate a 10‐year CVD score using the laboratory FRS equations. We, thus, ran an analysis with the office‐based FRS, using body mass index (height and weight were measured in the study) instead of cholesterol measures and self‐reported diabetes mellitus or presence of antidiabetic medication in lieu of glycosylated hemoglobin level. Data for these analyses were available for 1039 participants (99% of IMIAS women). We also examined AFB according to whether the participant did or did not have laboratory data; there were no significant differences according to missingness.
Regression diagnostics were performed to examine the validity of all linear regression models. This included performing log‐transformed analyses because of the right‐skewed distribution of the FRS. Data were analyzed with Stata, version 14.
Results
Sample Characteristics
Table 1 presents the participant characteristics according to AFB categories. Adolescent childbirth was much more prevalent in the Latin American study sites compared with the Albanian and Canadian sites. Of the 188 women who reported a first childbirth at <20 years old, 68.1% were from the Latin American sites. Women who were <20 years old at first childbirth had an overall higher prevalence of childhood economic and social adversity when compared with older AFB categories, especially the 25 to 29 years category. Young AFB women tended to have lower educational attainment and more often fell into the low‐income category. Women with a young AFB were also more likely to have given birth to ≥4 children (75.5%, compared with 37.6% of women with an AFB of 20–24 years, 13.1% of women with an AFB of 25–29 years, and 5.4% of women with an AFB of ≥30 years).
Table 1.
Distribution (or Means) of Sample Characteristics by AFB (N=905)a
| Characteristics | AFB, y | Nulliparous Women (N=100) | |||
|---|---|---|---|---|---|
| <20 (N=188) | 20–24 (N=355) | 25–29 (N=169) | ≥30 (N=93) | ||
| Study site, N (%) | |||||
| Kingston (Ontario, Canada) | 23 (12.2) | 48 (13.5) | 49 (29.0) | 27 (29.0) | 25 (25.0) |
| Saint‐Hyacinthe (Quebec, Canada) | 11 (5.9) | 71 (20.0) | 54 (32.0) | 18 (19.4) | 28 (28.0) |
| Tirana (Albania) | 26 (13.8) | 106 (29.9) | 32 (18.9) | 11 (11.8) | 13 (13.0) |
| Manizales (Colombia) | 75 (39.9) | 71 (20.0) | 18 (10.7) | 17 (18.3) | 17 (17.0) |
| Natal (Brazil) | 53 (28.2) | 59 (16.6) | 16 (9.5) | 20 (21.5) | 17 (17.0) |
| Age, mean (SD), y | 69.2 (2.9) | 69.1 (2.9) | 69.3 (2.8) | 68.9 (2.7) | 68.8 (2.7) |
| Childhood economic adversity, N (%)b | |||||
| No | 92 (48.9) | 192 (54.1) | 109 (64.9) | 51 (54.8) | 61 (61.0) |
| Yes | 96 (51.1) | 163 (45.9) | 59 (35.1) | 42 (45.2) | 39 (39.0) |
| Childhood social adversity, N (%)b | |||||
| No | 126 (67.0) | 267 (75.2) | 128 (76.2) | 70 (75.3) | 81 (81.0) |
| Yes | 62 (33.0) | 88 (24.8) | 40 (23.8) | 23 (24.7) | 19 (19.0) |
| Education site specific, N (%)c | |||||
| Low | 114 (60.6) | 144 (40.6) | 53 (31.4) | 27 (29.0) | 34 (34.0) |
| Medium | 54 (28.7) | 129 (36.3) | 56 (33.1) | 28 (30.1) | 31 (31.0) |
| High | 20 (10.6) | 82 (23.1) | 60 (35.5) | 38 (40.9) | 35 (35.0) |
| Income, N (%)d | |||||
| Poor | 81 (43.6) | 115 (32.7) | 57 (34.3) | 28 (31.1) | 30 (30.3) |
| Middle | 85 (45.7) | 168 (47.7) | 73 (44.0) | 41 (45.6) | 44 (44.4) |
| High | 20 (10.8) | 69 (19.6) | 36 (21.7) | 21 (23.3) | 25 (25.3) |
| Parity, N (%)e | |||||
| Nulliparous | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 100 (100.0) |
| 1–3 | 46 (24.5) | 221 (62.4) | 146 (86.9) | 88 (94.6) | 0 (0.0) |
| ≥4 | 142 (75.5) | 133 (37.6) | 22 (13.1) | 5 (5.4) | 0 (0.0) |
AFB indicates age at first childbirth.
AFB is missing for 3 participants.
One missing value.
Site specific, for women only (no laboratory included).
Twelve missing values.
Two missing values.
FRS by Sample Characteristics
The average FRS was 19.5 (SD, 13.1), with the lowest mean in Kingston and the highest mean in Tirana. Higher mean FRS scores indicate greater CVD risk. Older ages, childhood economic adversity, low education, younger AFB, and higher parity were significantly associated with higher mean FRS. No large differences in FRS were observed between individuals with or without childhood social adversities or between individuals with current low, middle, or high incomes in the bivariate analyses (Table 2).
Table 2.
FRS by Sample Characteristics (N=908)
| Characteristics | N | Mean | SD | P Value |
|---|---|---|---|---|
| Study site | ||||
| Kingston (Ontario, Canada) | 172 | 13.7 | 8.2 | <0.001 |
| Saint‐Hyacinthe (Quebec, Canada) | 183 | 14.3 | 8.3 | |
| Tirana (Albania) | 188 | 27.7 | 14.4 | |
| Manizales (Colombia) | 198 | 18.0 | 10.8 | |
| Natal (Brazil) | 167 | 23.6 | 16.1 | |
| Age in 2 categories, y | ||||
| 64–69 | 498 | 17.6 | 11.9 | <0.001 |
| 70–74 | 410 | 21.8 | 14.1 | |
| Childhood economic adversitya | ||||
| No | 507 | 18.5 | 12.7 | 0.012 |
| Yes | 400 | 20.7 | 13.6 | |
| Childhood social adversitya | ||||
| No | 674 | 19.7 | 13.5 | 0.546 |
| Yes | 233 | 19.0 | 11.9 | |
| Education site specificb | ||||
| Low | 372 | 21.0 | 13.5 | <0.001 |
| Medium | 301 | 20.2 | 14.1 | |
| High | 235 | 16.2 | 10.3 | |
| Incomec | ||||
| Poor | 313 | 18.5 | 12.1 | 0.218 |
| Middle | 412 | 20.2 | 13.5 | |
| High | 171 | 19.7 | 14.1 | |
| AFB, yd | ||||
| <20 | 188 | 23.2 | 14.9 | <0.001 |
| 20–24 | 355 | 20.8 | 13.8 | |
| 25–29 | 169 | 16.3 | 10.5 | |
| ≥30 | 93 | 17.7 | 11.7 | |
| Nulliparous | 100 | 14.8 | 9.0 | |
| Paritye | ||||
| Nulliparous | 100 | 14.8 | 9.0 | <0.001 |
| 1–3 | 501 | 19.0 | 12.5 | |
| ≥4 | 302 | 21.8 | 14.6 | |
AFB indicates age at first childbirth; and FRS, Framingham Risk Score.
One missing value.
Site specific, for women only.
Two missing values.
Three missing values.
Five missing values.
AFB and FRS
Table 3 presents the results of the multivariate models based on our conceptual model. Young AFB was positively associated with FRS. Statistically adjusting for age, study site, and childhood adversities, women with an AFB of <20 years had 3.1 points higher mean FRS (95% confidence interval [CI], 1.0–5.3) compared with women with an AFB of 20 to 24 years, 5.6 points higher mean FRS (95% CI, 3.1–8.2) compared with women with an AFB of 25 to 29 years, 3.9 points higher mean FRS (95% CI, 1.0–6.8) compared with women with an AFB of ≥30, and 6.7 points higher mean FRS (95% CI, 3.8–9.6) compared with nulliparous women (model 2) (Table 3). The association was only slightly attenuated in model 3, although the sample size also decreased. The results of model 3 are visually presented in Figure 2.
Table 3.
Linear Regression Models Presenting the Association of AFB With FRSs, Progressively Adjusting for Childhood and Adulthood Socioeconomic Risk Covariates
| Variable | Age and Site Only (Model 1) | Age, Site, and Childhood Adversities (Model 2) | Age, Site, Childhood Adversities, Education, and Income (Model 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (N=905) | (N=904) | (N=892) | |||||||
| Categories of AFB, y | |||||||||
| <20 | Ref | Ref | Ref | ||||||
| 20–24 | −3.15 | −5.26 | −1.03 | −3.14 | −5.26 | −1.01 | −2.92 | −5.09 | −0.76 |
| 25–29 | −5.64 | −8.19 | −3.10 | −5.63 | −8.19 | −3.07 | −5.29 | −7.93 | −2.65 |
| ≥30 | −3.91 | −6.84 | −0.99 | −3.90 | −6.84 | −0.96 | −3.12 | −6.15 | −0.08 |
| Nulliparous | −6.67 | −9.55 | −3.79 | −6.65 | −9.55 | −3.75 | −6.19 | −9.15 | −3.23 |
| Age (centered), y | 0.89 | 0.62 | 1.16 | 0.89 | 0.62 | 1.16 | 0.89 | 0.61 | 1.16 |
| Research site | |||||||||
| Kingston (Ontario, Canada) (ref) | Ref | Ref | Ref | ||||||
| Saint‐Hyacinthe (Quebec, Canada) | 1.44 | −0.99 | 3.87 | 1.42 | −1.02 | 3.86 | 1.74 | −0.83 | 4.32 |
| Tirana (Albania) | 13.53 | 11.09 | 15.98 | 13.57 | 11.09 | 16.06 | 14.09 | 11.53 | 16.64 |
| Manizales (Colombia) | 2.76 | 0.31 | 5.20 | 2.76 | 0.31 | 5.21 | 2.90 | 0.29 | 5.51 |
| Natal (Brazil) | 8.69 | 6.16 | 11.21 | 8.72 | 6.16 | 11.29 | 9.37 | 6.70 | 12.03 |
| Childhood economic adversity (no=ref) | −0.19 | −1.81 | 1.42 | −0.34 | −1.98 | 1.31 | |||
| Childhood social adversity (no=ref) | 0.20 | −1.61 | 2.01 | 0.12 | −1.70 | 1.95 | |||
| Education site specific | |||||||||
| Low (ref) | Ref | ||||||||
| Medium | −0.30 | −2.14 | 1.53 | ||||||
| High | −2.40 | −4.48 | −0.33 | ||||||
| Income | |||||||||
| Poor (ref) | Ref | ||||||||
| Middle | −1.76 | −3.64 | 0.12 | ||||||
| High | 0.09 | −2.34 | 2.52 | ||||||
| Constant | 17.61 | 17.62 | 18.60 | ||||||
Data are given as B (95% confidence interval). AFB indicates age at first childbirth; FRS, Framingham Risk Score; and Ref, reference.
Figure 2.
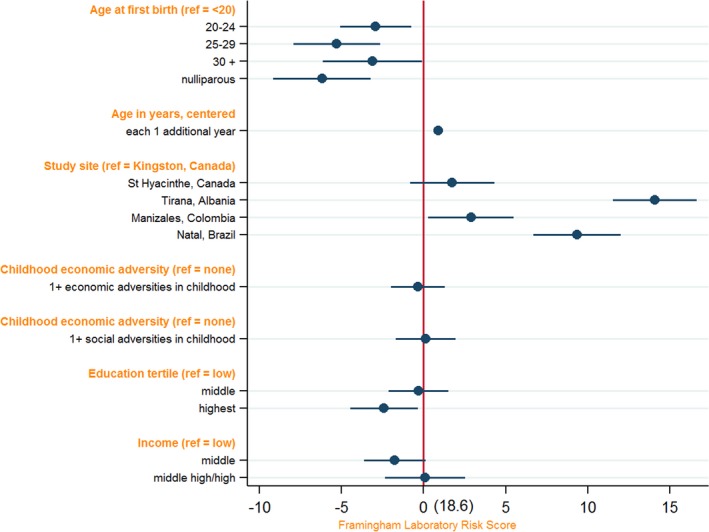
Association of age at first childbirth (AFB) and Framingham Risk Score (FRS); visualization of model 3 is displayed in Table 3. N=892. Ref indicates reference.
The association between AFB and FRS differed by study site (Table 4). For the Canadian sites, adjusting for age, study site, and childhood adversities, women with an AFB of <20 years had a higher mean FRS compared with all other groups, but only significantly so when compared with women with an AFB of ≥30 years (3.7 points; 95% CI, 0.1–7.3 points). Young AFB was strongly associated with higher FRS in Natal; compared with women with an AFB of 25 to 29 years, women with an AFB of <20 years had 11.5 (95% CI, 3.1–19.9) points higher mean FRS. In Tirana, we observed a J‐shaped association: those with an AFB of <20 years had 8.7 (95% CI, 1.4–16.0) points higher mean FRS compared with those with an AFB of 25 to 29 years, but 2.6 (95% CI, −12.5 to 7.4) points lower mean FRS compared with those with an AFB of ≥30 years. In both Tirana and Natal, mean FRS scores of the AFB <20 years group were also high compared with the nulliparous category (9.8 points in Tirana and 16.3 points in Natal; P<0.01). For Manizales, women with a young AFB had a higher FRS compared with the other groups, but this difference was small and not statistically significant.
Table 4.
Linear Regression Models Presenting the Association of AFB With FRS by Study Site, Adjusting for Childhood Socioeconomic Risk Covariates
| Variable | Canadian Sites | Tirana (Albania) | Manizales (Colombia) | Natal (Brazil) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N=353) | (N=188) | (N=198) | (N=165) | |||||||||
| Categories of AFB, y | ||||||||||||
| <20 | Ref | Ref | Ref | Ref | ||||||||
| 20–24 | −1.43 | −4.55 | 1.70 | −1.14 | −7.23 | 4.96 | −0.82 | −4.35 | 2.71 | −10.42 | −15.93 | −4.91 |
| 25–29 | −2.91 | −6.08 | 0.26 | −8.69 | −16.02 | −1.37 | −0.97 | −6.56 | 4.61 | −11.47 | −19.88 | −3.06 |
| ≥30 | −3.71 | −7.31 | −0.11 | 2.58 | −7.39 | 12.55 | −1.37 | −7.12 | 4.37 | −8.02 | −15.65 | −0.40 |
| Nulliparous | −3.47 | −7.00 | 0.06 | −9.83 | −19.25 | −0.41 | −2.33 | −8.09 | 3.42 | −16.33 | −24.51 | −8.16 |
| Age (centered), y | 0.59 | 0.26 | 0.92 | 0.90 | 0.21 | 1.58 | 0.72 | 0.21 | 1.22 | 2.15 | 1.29 | 3.00 |
| Research site (Kingston [Ontario, Canada]=ref)a | 0.97 | −0.75 | 2.70 | |||||||||
| Childhood economic adversity (no=ref) | 0.05 | −1.81 | 1.91 | −1.16 | −5.34 | 3.02 | 0.20 | −3.10 | 3.49 | 1.11 | −3.79 | 6.01 |
| Childhood social adversity (no=ref) | 1.76 | −0.16 | 3.69 | −0.05 | −6.12 | 6.03 | −0.58 | −4.36 | 3.20 | −2.32 | −7.12 | 2.48 |
| Constant | 15.40 | 30.95 | 18.61 | 30.88 | ||||||||
Data are given as B (95% confidence interval). AFB indicates age at first childbirth; FRS, Framingham Risk Score; and Ref, reference.
Only for Canadian site analysis. Coefficient shown is for Saint‐Hyacinthe (Quebec, Canada).
Parity, AFB, and FRS
Table 5 presents results for the combined AFB and parity categories, compared with nulliparous women, adjusted for study site, age, and childhood adversities. Although all parous groups have a higher mean FRS compared with nulliparous women, the difference between those with low parity (1–3) and high parity (≥4) is small when we examine across equal AFB groups (0.2 points in the 2 AFB ≥20 groups and 1.1 in the 2 AFB <20 groups). In contrast, there is a stark difference in mean FRS between the <20 versus ≥20 years AFB groups when we examine across equal parity categories. In the 1 to 3 parity category, women with an AFB of <20 years had 3.1 points higher mean FRS compared with women with an AFB of ≥20. In the ≥4 parity category, the difference between the 2 AFB groups is 4.0 points (Table 4).
Table 5.
Linear Regression Model Presenting the Association of Combinations of AFB and Parity Categories With FRSs
| Variable | B | 95% CI | |
|---|---|---|---|
| Parity and age | |||
| Nulliparous (N=100) | Ref | ||
| Parity 1–3, AFB <20 y (N=46) | 5.74 | 1.66 | 9.82 |
| Parity ≥4, AFB <20 y (N=142) | 6.82 | 3.71 | 9.93 |
| Parity 1–3, AFB ≥20 y (N=455) | 2.62 | 0.08 | 5.15 |
| Parity ≥4, AFB ≥20 y (N=160) | 2.78 | −0.22 | 5.77 |
| Age (centered), y | 0.88 | 0.60 | 1.15 |
| Research site | |||
| Kingston (Ontario, Canada) (ref) | Ref | ||
| Saint‐Hyacinthe (Quebec, Canada) | 1.46 | −0.97 | 3.89 |
| Tirana (Albania) | 13.87 | 11.43 | 16.32 |
| Manizales (Colombia) | 3.03 | 0.50 | 5.57 |
| Natal (Brazil) | 8.93 | 6.27 | 11.59 |
| Childhood economic adversity (no=ref) | −0.07 | −1.69 | 1.56 |
| Childhood social adversity (no=ref) | 0.25 | −1.56 | 2.06 |
| Constant | 10.78 | ||
N=903. AFB indicates age at first childbirth; CI, confidence interval; FRS, Framingham Risk Score; and Ref, reference.
Sensitivity Analysis
The sensitivity analysis using the office‐based FRS yielded results that were consistent with the results of the laboratory‐based FRS (Table 6); women <20 years at first childbirth had the greatest mean FRS across all models. We also performed a log‐transformed analysis because of the right‐skewed nature of the FRS measure, and these analyses yielded similar results, with slightly different effect sizes. For ease of interpretation, we only present the nontransformed model (log‐transformed results are available on request).
Table 6.
Linear Regression Models Presenting the Association of AFB With Office‐Based FRSs, Progressively Adjusting for Childhood and Adulthood Socioeconomic Risk Covariates
| Variable | Age and Site Only | Age, Site, and Childhood Adversities | Age, Site, Childhood Adversities, Education, and Income | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (N=1039) | (N=1038) | (N=1022) | |||||||
| Categories of AFB, y | |||||||||
| <20 | Ref | Ref | Ref | ||||||
| 20–24 | −2.34 | −4.65 | −0.03 | −2.21 | −4.53 | 0.11 | −1.89 | −4.26 | 0.48 |
| 25–29 | −5.41 | −8.14 | −2.68 | −5.14 | −7.88 | −2.40 | −4.57 | −7.42 | −1.73 |
| ≥30 | −3.48 | −6.67 | −0.29 | −3.30 | −6.49 | −0.10 | −2.44 | −5.75 | 0.88 |
| Nulliparous | −7.14 | −10.31 | −3.95 | −6.83 | −10.02 | −3.64 | −6.31 | −9.60 | −3.02 |
| Age (centered), y | 1.22 | 0.92 | 1.52 | 1.22 | 0.93 | 1.52 | 1.22 | 0.92 | 1.52 |
| Research site | |||||||||
| Kingston (Ontario, Canada) (ref) | Ref | Ref | Ref | ||||||
| Saint‐Hyacinthe (Quebec, Canada) | 1.99 | −0.62 | 4.61 | 1.93 | −0.69 | 4.56 | 2.63 | −0.18 | 5.43 |
| Tirana (Albania) | 11.87 | 9.23 | 14.51 | 11.89 | 9.21 | 14.58 | 12.34 | 9.56 | 15.12 |
| Manizales (Colombia) | −0.01 | −2.69 | 2.68 | 0.05 | −2.64 | 2.74 | 0.53 | −2.36 | 3.42 |
| Natal (Brazil) | 9.92 | 7.28 | 12.56 | 9.58 | 6.89 | 12.28 | 10.26 | 7.44 | 13.08 |
| Childhood economic adversity (no=ref) | 0.90 | −0.89 | −2.68 | 0.61 | −1.21 | 2.43 | |||
| Childhood social adversity (no=ref) | 1.36 | −0.62 | 3.34 | 1.35 | −0.66 | 3.35 | |||
| Education site specifica | |||||||||
| Low (ref) | Ref | ||||||||
| Medium | −1.38 | −3.39 | 0.64 | ||||||
| High | −3.11 | −5.38 | −0.83 | ||||||
| Income | |||||||||
| Poor (ref) | Ref | ||||||||
| Middle | −1.18 | −3.28 | 0.92 | ||||||
| High | 1.16 | −1.51 | 3.83 | ||||||
| Constant | 22.37 | 21.54 | 22.45 | ||||||
Data are given as B (95% confidence interval). AFB indicates age at first childbirth; FRS, Framingham Risk Score; and Ref, reference.
Site specific, for women only (no laboratory included).
Discussion
To the best of our knowledge, this is the first study to demonstrate, in postmenopausal women from multiple global settings, that adolescent childbirth is related to higher mean cardiovascular risk, as measured by FRS, compared with women who gave birth at later ages, and compared with nulliparous women. This association remained even after statistical adjustment for socioeconomic variables that may be on the pathway between AFB and FRS. Nulliparous women had the lowest average FRS. Among parous women, women with an AFB of 25 to 29 years had the lowest average FRS.
We also attempted to dissociate parity from AFB in examining the association with CVD risk. The analyses in which we used combination categories of AFB and parity allowed us to investigate differences related to parity for women in the same AFB category, and differences related to AFB for women in the same parity category. We observed small differences between women with a parity of 1 to 3 compared with women with a parity of ≥4 within equivalent AFB categories. We observed large differences between women with an AFB of <20 years compared with women with an AFB of ≥20 years within the same parity categories. All 4 AFB/parity categories had a higher mean FRS when compared with nulliparous women, suggesting that both AFB and nulliparity, but not increasing parity, drives the association with FRS.
Comparison With Other Studies
This is the first study to examine the association between adolescent childbirth and FRS as a measure of cardiovascular risk. As a result, we cannot compare our results with other studies examining the same outcome. Instead, we can compare our results with a limited number of studies examining the association between AFB and other CVD (risk) outcomes. Other studies observed positive associations with young AFB and overweight status,44 high blood pressure,16, 53, 54 and diabetes mellitus–related mortality.55 In previous work using IMIAS data, we reported statistically significant associations between AFB ≤18 years and participant‐reported physician‐diagnosed high blood pressure (odds ratio, 2.1) and diabetes mellitus (odds ratio, 1.9), and a marginal association with stroke (odds ratio, 1.9; P=0.06).24 Of 3 studies56, 57, 58 analyzing AFB and metabolic syndrome, another summary measure of CVD that includes many of the components that are also accounted for in the FRS, 2 observed a positive association of young AFB with metabolic syndrome.56, 57 In a recent analysis of the 1958 British birth cohort study, the authors observed a relationship between adolescent childbirth and many metabolic parameters assessed individually: higher body mass index, waist:hip ratio, blood pressure, triglycerides, glycosylated hemoglobin, and lower high‐density lipoprotein cholesterol.6 Finally, we recently conducted a systematic review on AFB and CVD events and mortality. The study findings were relatively consistent when young AFB was defined as 20 years or younger and pointed towards increased risk for CVD for women with a young AFB.18
In our analysis that compared combination categories of AFB and parity, we found an association between FRS and AFB, and between FRS and nulliparity, but not between FRS and greater lifetime parity. Although we do not know of any other studies that examined parity in relation to FRS, 10 studies57, 58, 59, 60, 61, 62, 63, 64, 65, 66 have examined associations between parity and metabolic syndrome. Nine studies58, 59, 60, 61, 62, 63, 64, 65, 66 observed a positive association between parity and metabolic syndrome. Of these 9 studies, 559, 60, 61, 64, 66 did not adjust for the participants' AFB. AFB is closely linked with parity23, 24; by not adjusting for AFB, differences in FRS may mistakenly be attributed to parity when, in fact, AFB is driving the association. The results from our analyses, in which we report on associations within strata of parity and AFB, suggest that it is AFB, and not increasing parity, that is associated with FRS. The 4 remaining studies58, 62, 63, 65 that still observed a positive association between parity and metabolic syndrome all adjusted for AFB, but as a continuous linear variable. This may result in residual confounding by AFB if there is a reverse J‐shaped association of AFB and CVD risk.
Mechanisms
Pregnancy has been described as a physiological “stress” test.67 Although some of the nulliparous women in our sample may have miscarried or terminated pregnancies, as a group, they would have experienced dramatically lower mean levels of pregnancy‐related complications and no, or much shorter, durations of stress tests on the body. These findings potentially explain the lower mean FRSs observed in the nulliparous group. Common pregnancy complications, such as gestational diabetes mellitus and hypertensive disorders, may reveal latent chronic disease or initiate a cascade of pathophysiological processes contributing to CVD.68 Preeclampsia, in particular, may be associated with increased later‐life risk of CVD.69 Meta‐analysis on the topic reports that women who experienced preeclampsia have nearly 3 times the risk of developing hypertension later in life and approximately double the risk of cardiovascular and cerebrovascular events.69 It is unclear whether preeclampsia represents an independent causal risk factor for CVD by inducing systematic endothelial damage that manifests later in life or whether preeclampsia and CVD share a common underlying risk factor.69 Early childbearing is a risk factor for preeclampsia.70 Preeclampsia may, therefore, be on the pathway between early childbearing and CVD risk later in life.
Our findings warrant future research investigating the pathways that may explain the observed associations. Selection mechanisms, and mediation through physiological and social pathways, could explain the observed association between AFB and FRS. There may be unobserved characteristics in childhood and puberty, such as a risk‐seeking personality that “selects” women into both adolescent pregnancies and risky health behaviors; these negatively influence lifetime cardiovascular health.33, 71 Alternatively, or in combination, greater gain and retention of body weight, fewer career opportunities, higher stress levels, preeclampsia and other pregnancy complications, smaller social networks, and less social support are examples of possible mediators between early AFB and higher FRSs.25, 26, 31, 32, 33, 69 The lack of a uniform association across the study sites supports the possibility of social factors mediating the observed associations. Possibly, different familial, social, and governmental support across study sites may alter the association between AFB and FRS.
Limitations
Study participants were asked to report retrospectively about the reproductive exposures of AFB and parity, which may have introduced recall bias. However, both are major life events that are unlikely to have been forgotten. Furthermore, all study participants passed the orientation scale of the Leganes cognitive test for dementia, limiting the possibility that cognitive decline influenced recall.37, 38 More important, selective survival may have influenced our results, likely reducing the strength of association observed.72, 73, 74 If exposure of early AFB is associated with higher CVD risk, a larger proportion of high‐risk individuals may have “selected out” of the sample by not surviving to recruitment. Sampled women were between 64 and 75 years; in places like Natal, Brazil, these women have nearly doubled their life expectancy at birth.74
The FRS equation that we used is typically used to predict 10‐year CVD risk in a clinical setting for individual patients with no CVD diagnosed. Our analyses examining the association of AFB, parity, and cardiovascular risk, measured by FRS, used a sample that contained participants with and without CVD. We are not unique in using the FRS as a composite measure of CVD risk; a similar approach was also taken by the CARMELA (Cardiovascular Risk Factor Multiple Evaluation in Latin America) study when assessing prevalent cardiovascular risk in a population of ≈11 500 participants from 7 Latin American cities.75 Although it is inappropriate to interpret the FRSs obtained in these analyses for prediction purposes, the FRS does provide a useful and elegant composite measure of the classic risk factors for CVD. Finally, laboratory‐based FRSs were not available for 13% of the women who participated in the study. However, the sensitivity analyses with office‐based FRS, which were available for >99% of study participants, revealed markedly similar results.
Conclusions
Adolescent childbirth was frequent in our sample of older adults, especially among those from Latin America. Adolescent childbearing remains an issue of global importance, with an estimated 11% of births worldwide occurring among adolescents.76 Besides the known risks of adolescent childbirth (eg, delivery complications and stillbirth),76 our study suggests that young AFBs are associated with long‐term CVD risk. As highlighted above, more work is needed to carve out the pathways by which AFB is associated with CVD. Although we cannot accredit causal inference, at the least, young AFB can serve as a risk marker. Identification of this higher‐risk group could prove useful for clinicians, because women who were adolescent mothers may benefit from earlier and increased cardiovascular screening to reduce the incidence of CVD events. The clear dissociation of AFB and elevated parity in the association with CVD suggests that nulliparity and AFB, rather than increasing parity, drive the association with CVD risk. This finding provides a possible explanation for the conflicting results that have been reported in the literature to date on parity and CVD.
Sources of Funding
This study was supported by the Canadian Institutes of Health Research and by the Fogarty International Center of the National Institutes of Health (award R21TW010466). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Canadian Institutes of Health Research.
Disclosures
None.
Acknowledgments
We thank Dr Maria‐Victoria Zunzunegui for critical review of the article and the entire IMIAS (International Mobility in Aging Study) research team, without whom this study would never have been possible.
(J Am Heart Assoc. 2017;6:e007058 DOI: 10.1161/JAHA.117.007058.)29092844
References
- 1. GBD 2013 Mortality and Causes of Death Collaborators . Global, regional, and national age–sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ness R, Harris T, Cobb J, Flegal KM, Kelsey J, Balanger A, Stunkard AJ, D'Agostino RB. Number of pregnancies and the subsequent risk of cardiovascular disease. N Engl J Med. 1993;328:1528–1533. [DOI] [PubMed] [Google Scholar]
- 3. Canoy D, Beral V, Balkwill A, Wright FL, Kroll ME, Reeves GK, Green J, Cairns BJ; Million Women Study Collaborators . Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation. 2015;131:237–244. [DOI] [PubMed] [Google Scholar]
- 4. de Kleijn MJJ, van der Schouw YT, van der Graaf Y. Reproductive history and cardiovascular disease risk in postmenopausal women: a review of the literature. Maturitas. 1999;33:7–36. [DOI] [PubMed] [Google Scholar]
- 5. Mishra GD, Anderson D, Schoenaker DA, Adami H‐O, Avis NE, Brown D, Bruinsma F, Brunner E, Cade JE, Crawford SL, Dobson AJ, Elliott J, Giles GG, Gold EB, Hayashi K, Kuh D, Lee KA, Lee JS, Melby MK, Mizunuma H, Sievert LL, Weiderpass E. InterLACE: a new international collaboration for a life course approach to women's reproductive health and chronic disease events. Maturitas. 2013;74:235–240. [DOI] [PubMed] [Google Scholar]
- 6. Lacey RE, Kumari M, Sacker A, McMunn A. Age at first birth and cardiovascular risk factors in the 1958 British birth cohort. J Epidemiol Community Health. 2017;71:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ness R, Schotland H, Flegal K, Schofer F. Reproductive history and coronary heart disease risk in women. Epidemiol Rev. 1994;16:298–314. [DOI] [PubMed] [Google Scholar]
- 8. Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw K‐T, Wareham NJ, Ong KK. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94:4953–4960. [DOI] [PubMed] [Google Scholar]
- 9. Charalampopoulos D, McLoughlin A, Elks CE, Ong KK. Age at menarche and risks of all‐cause and cardiovascular death: a systematic review and meta‐analysis. Am J Epidemiol. 2014;180:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kivimäki M, Lawlor DA, Smith GD, Elovainio M, Jokela M, Keltikangas‐Järvinen L, Vahtera J, Taittonen L, Juonala M, Vikari JS, Raitakari OT. Association of age at menarche with cardiovascular risk factors, vascular structure, and function in adulthood: the Cardiovascular Risk in Young Finns study. Am J Clin Nutr. 2008;87:1876–1882. [DOI] [PubMed] [Google Scholar]
- 11. Westendorp R, Kirkwood T. Human longevity at the cost of reproductive success. Nature. 1998;396:743–746. [DOI] [PubMed] [Google Scholar]
- 12. Doblhammer G. Reproductive history and mortality later in life: a comparative study of England and Wales and Austria. Popul Stud (Camb). 2000;54:169–176. [DOI] [PubMed] [Google Scholar]
- 13. Otterblad Olausson P, Haglund B, Ringbäck Weitoft G, Cnattingius S. Premature death among teenage mothers. BJOG. 2004;111:793–799. [DOI] [PubMed] [Google Scholar]
- 14. Webb RT, Marshall CE, Abel KM. Teenage motherhood and risk of premature death: long‐term follow‐up in the ONS Longitudinal Study. Psychol Med. 2011;41:1867–1877. [DOI] [PubMed] [Google Scholar]
- 15. Hardy R, Lawlor DA, Black S, Mishra GD, Kuh D. Age at birth of first child and coronary heart disease risk factors at age 53 years in men and women: British birth cohort study. J Epidemiol Community Health. 2008;63:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lind JM, Hennessy A, Chiu CL. Association between a womanʼs age at first birth and high blood pressure. Medicine (Baltimore). 2015;94:e697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grundy E, Read S. Pathways from fertility history to later life health: results from analyses of the English Longitudinal Study of Ageing. Demogr Res. 2015;32:107–146. [Google Scholar]
- 18. Rosendaal NTA, Pirkle CM. Age at first birth and risk of later‐life cardiovascular disease: a systematic review of the literature, its limitation, and recommendations for future research. BMC Public Health. 2017;17:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rich‐Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women's future cardiovascular health: an underused opportunity to improve women's health? Epidemiol Rev. 2014;36:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Green A, Beral V, Moser K. Mortality in women in relation to their childbearing history. BMJ. 1988;297:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parikh NI, Cnattingius S, Dickman PW, Mittleman MA, Ludvigsson JF, Ingelsson E. Parity and risk of later‐life maternal cardiovascular disease. Am Heart J. 2010;159:215–221.e6. [DOI] [PubMed] [Google Scholar]
- 22. Steenland K, Lally C, Thun M. Parity and coronary heart disease among women in the American Cancer Society CPS II population. Epidemiology. 1996;7:641–643. [DOI] [PubMed] [Google Scholar]
- 23. Câmara SMA, Pirkle C, Moreira MA, Vieira MCA, Vafaei A, Maciel ÁCC. Early maternal age and multiparity are associated to poor physical performance in middle‐aged women from Northeast Brazil: a cross‐sectional community based study. BMC Womens Health. 2015;15:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pirkle CM, de Albuquerque Sousa ACP, Alvarado B, Zunzunegui M‐V. Early maternal age at first birth is associated with chronic diseases and poor physical performance in older age: cross‐sectional analysis from the International Mobility in Aging Study. BMC Public Health. 2014;14:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gigante DP, Rasmussen KM, Victora CG. Pregnancy increases BMI in adolescents of a population‐based birth cohort. J Nutr. 2005;135:74–80. [DOI] [PubMed] [Google Scholar]
- 26. Groth SW. The long‐term impact of adolescent gestational weight gain. Res Nurs Health. 2008;31:108–118. [DOI] [PubMed] [Google Scholar]
- 27. Gunderson EP, Schreiber G, Striegel‐Moore R, Hudes M, Daniels S, Biro FM, Crawford PB. Pregnancy during adolescence has lasting adverse effects on blood lipids: a 10‐year longitudinal study of black and white females. J Clin Lipidol. 2012;6:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Howie LD, Parker JD, Schoendorf KC. Excessive maternal weight gain patterns in adolescents. J Am Diet Assoc. 2003;103:1653–1657. [DOI] [PubMed] [Google Scholar]
- 29. Kim JH, Jung Y, Kim SY, Bae HY. Impact of age at first childbirth on glucose tolerance status in postmenopausal women: the 2008–2011 Korean National Health and Nutrition Examination Survey. Diabetes Care. 2014;37:671–677. [DOI] [PubMed] [Google Scholar]
- 30. Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA. 2005;294:2751–2757. [DOI] [PubMed] [Google Scholar]
- 31. Hoffman SD, Foster EM, Furstenberg FF Jr. Reevaluating the costs of teenage childbearing. Demography. 1993;30:1–13. [PubMed] [Google Scholar]
- 32. Ermisch J. Does a “teen‐birth” have longer‐term impacts on the mother? suggestive evidence from the British Household Panel Study [Internet]. ISER Working Paper Series; 2003. http://www.econstor.eu/handle/10419/91969. Accessed February 10, 2016.
- 33. Steinburg L. Age of Opportunity: Lessons from the New Science of Adolescence: Eamon Dolan/Houghton Mifflin. Boston, MA, USA; 2014. [Google Scholar]
- 34. Gruenewald TL, Karlamangla AS, Hu P, Stein‐Merkin S, Crandall C, Koretz B, Seeman TE. History of socioeconomic disadvantage and allostatic load in later life. Soc Sci Med. 2012;74:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gustafsson PE, Janlert U, Theorell T, Westerlund H, Hammarstrom A. Socioeconomic status over the life course and allostatic load in adulthood: results from the Northern Swedish Cohort. J Epidemiol Community Health. 2011;65:986–992. [DOI] [PubMed] [Google Scholar]
- 36. United Nations Development Programme . ed. Work for Human Development. New York, NY: United Nations Development Programme; 2015:272. (Human development report). [Google Scholar]
- 37. Caldas VV, Zunzunegui MV, Freire Ado NF, Guerra RO. Translation, cultural adaptation and psychometric evaluation of the Leganés cognitive test in a low educated elderly Brazilian population. Arq Neuropsiquiatr. 2012;70:22–27. [DOI] [PubMed] [Google Scholar]
- 38. Zunzunegui MV, Gutiérrez Cuadra P, Béland F, Del Ser T, Wolfson C. Development of simple cognitive function measures in a community dwelling population of elderly in Spain. Int J Geriatr Psychiatry. 2000;15:130–140. [DOI] [PubMed] [Google Scholar]
- 39. Zunzunegui MV, Alvarado BE, Guerra R, Gómez JF, Ylli A, Guralnik JM; for the IMIAS research group . The mobility gap between older men and women: the embodiment of gender. Arch Gerontol Geriatr. 2015;61:140–148. [DOI] [PubMed] [Google Scholar]
- 40. Framingham Heart Study [Internet]. https://www.framinghamheartstudy.org. Accessed September 1, 2016.
- 41. D'Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 42. American Diabetes Association . Classification and diagnosis of diabetes in standards of medical care in diabetes. Diabetes Care. 2016;39:S13–S22. [DOI] [PubMed] [Google Scholar]
- 43. Sousa AC, Guerra RO, Thanh Tu M, Phillips S, Guralnik JM, Zunzunegui MV. Lifecourse adversity and physical performance across countries among men and women aged 65–74. PLoS One. 2014;9:e102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lopez‐Arana S, Burdorf A, Avendano M. Trends in overweight by educational level in 33 low‐ and middle‐income countries: the role of parity, age at first birth and breastfeeding: overweight in low‐ and middle‐income countries. Obes Rev. 2013;14:806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hobcraft J, Kiernan K. Childhood poverty, early motherhood and adult social exclusion. Br J Sociol. 2001;52:495–517. [DOI] [PubMed] [Google Scholar]
- 46. Hillis SD, Anda RF, Dube SR, Felitti VJ, Marchbanks PA, Marks JS. The association between adverse childhood experiences and adolescent pregnancy, long‐term psychosocial consequences, and fetal death. Pediatrics. 2004;113:320–327. [DOI] [PubMed] [Google Scholar]
- 47. Bleil ME, Adler NE, Appelhans BM, Gregorich SE, Sternfeld B, Cedars MI. Childhood adversity and pubertal timing: understanding the origins of adulthood cardiovascular risk. Biol Psychol. 2013;93:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Non AL, Rewak M, Kawachi I, Gilman SE, Loucks EB, Appleton AA, Roman JC, Buka SL, Kubzansky LD. Childhood social disadvantage, cardiometabolic risk, and chronic disease in adulthood. Am J Epidemiol. 2014;180:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Campbell JA, Walker RJ, Egede LE. Associations between adverse childhood experiences, high‐risk behaviors, and morbidity in adulthood. Am J Prev Med. 2016;50:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Su S, Jimenez MP, Roberts CTF, Loucks EB. The role of adverse childhood experiences in cardiovascular disease risk: a review with emphasis on plausible mechanisms. Curr Cardiol Rep. 2015;17:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cois A, Ehrlich R. Analysing the socioeconomic determinants of hypertension in South Africa: a structural equation modelling approach. BMC Public Health. 2014;14:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health. 1992;82:816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kharazmi E, Kaaja R, Fallah M, Luoto R. Pregnancy‐related factors and the risk of isolated systolic hypertension. Blood Press. 2007;16:50–55. [DOI] [PubMed] [Google Scholar]
- 54. Park JS, Jung I, Youn J‐C, Cho HY. Impact of adolescent pregnancy on hypertension in postmenopausal women. J Hypertens. 2016;34:47–53. [DOI] [PubMed] [Google Scholar]
- 55. Vandenheede H, Deboosere P, Gadeyne S, De Spiegelaere M. The associations between nationality, fertility history and diabetes‐related mortality: a retrospective cohort study in the Brussels‐Capital Region (2001–2005). J Public Health (Oxf). 2011;34:100–107. [DOI] [PubMed] [Google Scholar]
- 56. Sim JH, Chung D, Lim JS, Lee MY, Chung CH, Shin JY, Huh JH. Maternal age at first delivery is associated with the risk of metabolic syndrome in postmenopausal women: from 2008–2010 Korean National Health and Nutrition Examination Survey. PLoS One. 2015;10:e0127860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cho GJ, Park HT, Shin JH, Kim T, Hur JY, Kim YT, Lee KW, Kim SH. The relationship between reproductive factors and metabolic syndrome in Korean postmenopausal women: Korea National Health and Nutrition Survey 2005. Menopause. 2009;16:998–1003. [DOI] [PubMed] [Google Scholar]
- 58. Moradi S, Zamani F, Pishgar F, Ordookhani S, Nateghi N, Salehi F. Parity, duration of lactation and prevalence of maternal metabolic syndrome: a cross‐sectional study. Eur J Obstet Gynecol Reprod Biol. 2016;201:70–74. [DOI] [PubMed] [Google Scholar]
- 59. Vladutiu CJ, Siega‐Riz AM, Sotres‐Alvarez D, Stuebe AM, Ni A, Tabb KM, Gallo LC, Potter JE, Heiss G. Parity and components of the metabolic syndrome among US Hispanic/Latina women results from the Hispanic Community Health Study/Study of Latinos. Circ Cardiovasc Qual Outcomes. 2016;9:S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu M, He Y, Jiang B, Wu L, Wang J, Yang S, Wang Y, Li X. Association between reproductive variables and metabolic syndrome in Chinese community elderly women. Arch Gerontol Geriatr. 2016;63:78–84. [DOI] [PubMed] [Google Scholar]
- 61. Wu J, Xu G, Shen L, Zhang Y, Song L, Yang S, Wang Y, Li X. Parity and risk of metabolic syndrome among Chinese women. J Womens Health. 2015;24:602–607. [DOI] [PubMed] [Google Scholar]
- 62. Akter S, Jesmin S, Rahman MM, Islam MM, Khatun MT, Yamaguchi N, Akashi H, Mizutani T. Higher gravidity and parity are associated with increased prevalence of metabolic syndrome among rural Bangladeshi women. PLoS One. 2013;8:e68319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lao XQ, Thomas GN, Jiang CQ, Zhang WS, Yin P, Schooling M, Heys M, Leung GM, Adab P, Cheng KK, Lam TH. Parity and the metabolic syndrome in older Chinese women: the Guangzhou Biobank Cohort Study. Clin Endocrinol (Oxf). 2006;65:460–469. [DOI] [PubMed] [Google Scholar]
- 64. Gunderson EP, Jacobs DR, Chiang V, Lewis CE, Tsai A, Quesenberry CP Jr, Sidney S. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol. 2009;201:177.e1–177.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mousavi E, Gharipour M, Tavassoli A, Sadri GH, Sarrafzadegan N. Multiparity and risk of metabolic syndrome: Isfahan Healthy Heart Program. Metab Syndr Relat Disord. 2009;7:519–524. [DOI] [PubMed] [Google Scholar]
- 66. Cohen A, Pieper CF, Brown AJ, Bastian LA. Number of children and risk of metabolic syndrome in women. J Womens Health. 2006;15:763–773. [DOI] [PubMed] [Google Scholar]
- 67. Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rich‐Edwards JW, McElrath TF, Karumanchi SA, Seely EW. Breathing life into the lifecourse approach: pregnancy history and cardiovascular disease in women. Hypertension. 2010;56:331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre‐eclampsia: systematic review and meta‐analysis. Eur J Epidemiol. 2013;28:1–19. [DOI] [PubMed] [Google Scholar]
- 70. Ganchimeg T, Ota E, Morisaki N, Laopaiboon M, Lumbiganon P, Zhang J, Yamdamsuren B, Temmerman M, Say L, Tuncalp O, Vogel JP, Souza JP, Mori R; WHO, Multicountry Survey on Maternal Newborn Health Research Network . Pregnancy and childbirth outcomes among adolescent mothers: a World Health Organization multicountry study. BJOG. 2014;121:40–48. [DOI] [PubMed] [Google Scholar]
- 71. Mohsin M, Bauman AE. Socio‐demographic factors associated with smoking and smoking cessation among 426,344 pregnant women in New South Wales, Australia. BMC Public Health. 2005;5:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Markides KS, Machalek R. Selective survival, aging and society. Arch Gerontol Geriatr. 1984;3:207–222. [DOI] [PubMed] [Google Scholar]
- 73. Willson AE, Shuey KM, Elder GH Jr. Cumulative advantage processes as mechanisms of inequality in life course health. Am J Sociol. 2007;112:1886–1924. [Google Scholar]
- 74. Oliveira BS, Zunzunegui MV, Quinlan J, Batistuzzo de Medeiros SR, Thomasini RL, Guerra RO. Lifecourse adversity and telomere length in older women from Northeast Brazil. Rejuvenation Res. 2017. Available at: http://online.liebertpub.com/doi/10.1089/rej.2017.1937. Accessed October 18, 2017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 75. Schargrodsky H, Hernández‐Hernández R, Champagne BM, Silva H, Vinueza R, Silva Ayçaguer LC, Touboul PJ, Boissonnet CP, Escobedo J, Pellegrini F, Macchia A, Wilson E. CARMELA: assessment of cardiovascular risk in seven Latin American cities. Am J Med. 2008;121:58–65. [DOI] [PubMed] [Google Scholar]
- 76. WHO . WHO|Adolescent Pregnancy [Internet]. http://www.who.int/mediacentre/factsheets/fs364/en. Accessed June 21, 2017.


