Figure 14.
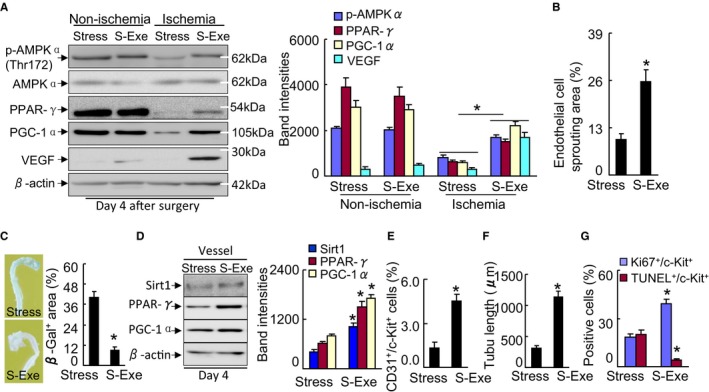
GLP‐1R activation ameliorated targeted protein levels in stressed mice. A, Representative immunoblots and quantitative data show the levels of p‐AMPKα, PPAR‐γ, PGC‐1α, and VEGF of the muscles of stressed mice. B, Quantification of aortic endothelial sprouting area in both experimental groups. C, Representative images and quantitative data show that exenatide improved aortic endothelial senescence (n=4). D, Representative immunoblots and quantitative data show the levels of PPAR‐γ and PGC‐1α of the vessels of stressed mice (n=3). E, Quantification of the numbers of peripheral blood CD31+/c‐Kit+ EPC numbers (per 2×104 cells). F, BM‐derived c‐Kit+ cells (5×103 cells) were cultured on growth factor–reduced Matrigel for 24 h and then the tubule lengths were calculated. G, BM‐derived c‐Kit+ cells (5×103 cells) were seeded on a 4‐chamber culture slide and cultured for 72 h. The cells were subjected to proliferation and apoptosis induced by H2O2 (250 μmol/L) assays using Ki67 antibody or a TUNEL staining kit, respectively (n=5–6). Data are mean±SE. *P<0.01 by Student unpaired t test or ANOVA and Tukey's post hoc tests. BM, indicates bone marrow; EPC, endothelial progenitor cell; GLP‐1R, glucagon‐like peptide‐1 receptor; p‐AMPKα, phospho‐AMP‐activated protein kinase α; PGC‐1α, PPAR‐γ co‐activator 1α; PPAR‐γ, peroxisome proliferator‐activated receptor‐γ; TUNEL, TdT‐mediated dUTP nick‐end labeling; VEGF, vascular endothelial growth factor.
