Abstract
Background
The DENERHTN (Renal Denervation for Hypertension) trial confirmed the efficacy of renal denervation (RDN) in lowering daytime ambulatory systolic blood pressure when added to standardized stepped‐care antihypertensive treatment (SSAHT) for resistant hypertension at 6 months.
Methods and Results
This post hoc exploratory analysis assessed the impact of abdominal aortic calcifications (AAC) on the hemodynamic and renal response to RDN at 6 months. In total, 106 patients with resistant hypertension were randomly assigned to RDN plus SSAHT or to the same SSAHT alone (control group). Total AAC volume was measured, with semiautomatic software and blind to randomization, from the aortic hiatus to the iliac bifurcation using the prerandomization noncontrast abdominal computed tomography scans of 90 patients. Measurements were expressed as tertiles. The baseline‐adjusted difference in the change in daytime ambulatory systolic blood pressure from baseline to 6 months between the RDN and control groups was −10.1 mm Hg (P=0.0462) in the lowest tertile and −2.5 mm Hg (P=0.4987) in the 2 highest tertiles of AAC volume. Estimated glomerular filtration rate remained stable at 6 months for the patients in the lowest tertile of AAC volume who underwent RDN (+2.5 mL/min per 1.73 m2) but decreased in the control group (−8.0 mL/min per 1.73 m2, P=0.0148). In the 2 highest tertiles of AAC volume, estimated glomerular filtration rate decreased similarly in the RDN and control groups (P=0.2640).
Conclusions
RDN plus SSAHT resulted in a larger decrease in daytime ambulatory systolic blood pressure than SSAHT alone in patients with a lower AAC burden than in those with a higher AAC burden. This larger decrease in daytime ambulatory systolic blood pressure was not associated with a decrease in estimated glomerular filtration rate.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01570777.
Keywords: antihypertensive therapy/sympathetic nervous system, aortic calcification, aortic disease, atherosclerosis, renal denervation, resistant hypertension
Subject Categories: Hypertension, Treatment, Vascular Disease
Clinical Perspective
What Is New?
The beneficial blood pressure–lowering effect of renal denervation in addition to standardized stepped‐care antihypertensive treatment is more pronounced in patients with resistant hypertension and a low abdominal aortic calcification burden and is accompanied by stability of estimated glomerular filtration rate.
Conversely, those with a high abdominal aortic calcification burden do not seem to benefit from an additive effect of renal denervation and standardized stepped‐care antihypertensive treatment.
What Are the Clinical Implications?
The ambulatory blood pressure reduction with renal denervation is clinically meaningful in patients with low abdominal aortic calcification burden and may help to decrease cardiovascular morbidity and mortality if maintained long term.
Renal denervation may be more effective in younger patients with a low abdominal aortic calcification burden, justifying new clinical trials for milder forms of hypertension.
Introduction
The DENERHTN (Renal Denervation for Hypertension) trial showed that renal denervation (RDN) added to standardized stepped‐care antihypertensive treatment (SSAHT) lowered blood pressure (BP) more effectively than the same SSAHT alone at 6 months in patients with resistant hypertension confirmed by ambulatory BP monitoring.1 Considerable between‐patient variability in the BP response was observed in both the RDN and control groups, but this variability was greater in the RDN group than in the control group. Male sex, baseline daytime ambulatory systolic BP (SBP), baseline nighttime ambulatory SBP and its standard deviation, changes in daytime ambulatory heart rate, low treatment score, high adherence to SSHAT were independently associated with the BP response, whereas ethnic origin and the number of ablations were not.1, 2, 3 Nevertheless, identification of predictive factors of BP response to RDN is still necessary to facilitate the selection of patients likely to benefit most from this invasive procedure.4, 5
The presence of abdominal aortic calcifications (AAC) is associated with greater aortic stiffness6, 7 and a poorer hemodynamic response to antihypertensive drugs.8 However, it is unknown whether the loss of the viscoelastic and biomechanical properties of the aorta associated with AAC also affects the systemic and renal hemodynamic response to RDN in patients with resistant hypertension. A poorer BP‐lowering response to RDN has been reported in patients with isolated systolic hypertension (ISH), a condition associated with an increase in arterial stiffness.9, 10 In this post hoc analysis of the DENERHTN study, we hypothesized that the presence of AAC may influence the overall hemodynamic and renal response to RDN plus SSAHT and to SSAHT alone.
Patients and Methods
Study Design
The design of the DENERHTN trial has been described elsewhere and thus will be summarized only briefly.1 In this PROBE (prospective randomized open blind end point) trial, patients with confirmed essential resistant hypertension; suitable renal artery anatomy, as evaluated by renal computed tomography (CT) angiogram (n=99) or magnetic resonance imaging (MRI; n=7); and an estimated glomerular filtration rate (eGFR) ≥40 mL/min per 1.73 m2 were randomly assigned, in a 1:1 ratio, to the RDN‐plus‐SSAHT or the SSAHT‐alone group. Before randomization, all eligible patients received 1.5 mg slow‐release indapamide, 10 mg ramipril (or 300 mg irbesartan), and 10 mg amlodipine (or 5 mg) daily for 4 weeks, for the confirmation of resistance to treatment by ambulatory BP monitoring. After randomization, we sequentially added 25 mg spironolactone, 10 mg bisoprolol, 5 mg prazosin, and 1 mg rilmenidine daily from months 2 to 5 in both groups if home BP was ≥135/85 mm Hg. We performed a median of 11 (interquartile range: 10–12) renal nerve ablations per patient, with a Symplicity (Medtronic) single‐electrode radiofrequency catheter, 2 to 4 weeks after randomization. The primary end point was change in daytime ambulatory SBP at 6 months. The study was approved by the Comité de Protection des Personnes Ile de France VII (ClinicalTrials.gov identifier: NCT01570777). All participants provided written informed consent for participation in the study.
CT Scans of the Abdominal Aorta
We used the renal and abdominal CT angiograms performed before randomization to measure AAC volume. The same imaging parameters were used for all CT scan acquisitions: 2‐mm slices, 120‐kV tube voltage, 250‐mAs tube current, and pitch 0.984. The reconstruction parameters for axial slices were an effective section thickness of 0.8 mm, a 0.4‐mm increment, and an adapted field of view.
Only CT scans of the abdominal aorta, not MRI, were used to measure AAC volume. We obtained renal and abdominal 64‐slice CT scans before and after the injection of contrast agent, in the head‐to‐foot direction. Images were obtained for the whole abdominal aorta, from the diaphragm to the iliac bifurcation, and were sent to the imaging core laboratory.
AACs were detected with a level‐set method constrained by the Hounsfield units of the delineated structures,11 as a plaque ≥1 mm2 in area with a density of >130 Hounsfield units from the aortic hiatus to the iliac bifurcation on noncontrast CT images (Figure 1A, 1B, 1B′, 1C, and 1C′). The total volume of the delineated AAC was then automatically calculated with semiautomatic software (Workstation AW server viewer, version 2.0; GE Medical Systems). Volumes of AAC were not adjusted for calcium density. Patients were classified into tertiles by AAC volume (Figure 1D through 1I). All measurements were performed by a trained radiologist blinded to randomization and the 6‐month BP results. Within‐ and between‐ observer reproducibility was assessed for AAC volume in a sample of 20 randomly selected patients.
Figure 1.
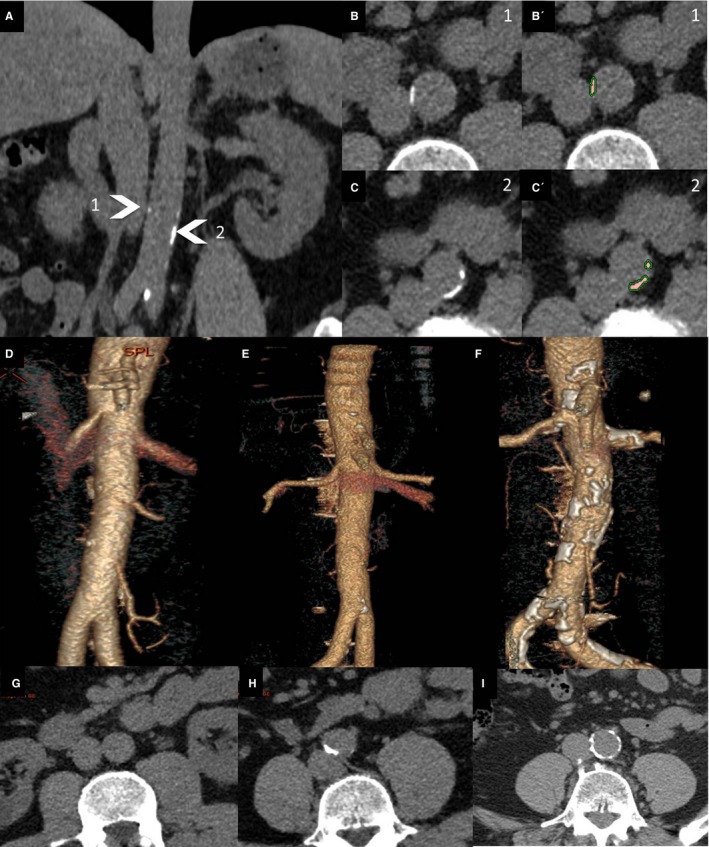
Example of aortic calcification segmentation: frontal view of the abdominal aorta (A) and corresponding cross‐sectional reconstructions at 2 levels: level 1 (B and B′) and level 2 (C and C′). Native images (B and C) and segmented images (B′ and C′) are provided with an overlay for the calcifications. Three‐dimensional volume reconstruction for the abdominal aorta of patients, by tertile for abdominal aortic calcification, is shown (D through F), with the results for axial slices (G through I).
Hemodynamic Assessments
Supine office BP, 7‐day seated home BP, and 24‐hour ambulatory BP (Spacelabs 90207 monitor; Spacelabs Healthcare) measurements were performed before randomization and at 6 months, as described previously.1 ISH at baseline was defined as a daytime SBP ≥135 mm Hg and a daytime diastolic BP <85 mm Hg. Aortic stiffness was estimated through carotid–femoral pulse wave velocity (PWV) measured with a Sphygmocor device (AtCor Medical Pty. Ltd; n=82) or with a Complior device (Alam Medical; n=8). PWV was measured over 30 minutes in supine position along the descending thoracoabdominal aorta by the validated foot‐to‐foot velocity method (mean of 3 measurements).12 Real travel distance was used (0.8×direct travel distance) to assess PWV, as recommended.12 PWV measured with a Complior device was converted using the following formula: PWV Sphygmocor=2.335+1.363×PWV Complior.13
Biological Assessments
We estimated eGFR from plasma creatinine concentration, using the MDRD (Modification of Diet in Renal Disease) formula.14 Full adherence to SSAHT at 6 months was assessed by determinations of drug concentrations in plasma or urine, as described previously.2
Statistical Analyses
We compared the baseline characteristics of the patients from each tertile for AAC volume by ANOVA and Kruskal–Wallis tests, as appropriate, for continuous variables and with the χ2 or Fisher exact test for categorical variables. Paired comparisons were made between tertiles for AAC volume, with Tukey correction for multiple testing on continuous variables and Bonferroni correction for multiple testing on categorical variables.
We compared the treatment groups (RDN versus control group) at baseline using the unpaired t tests or Wilcoxon rank‐sum tests for continuous variables, or the χ2 or Fisher exact tests for categorical variables. The baseline characteristics of the patients in the second and third tertiles for AAC volume did not differ significantly; therefore, we combined the data for the patients in these 2 tertiles to assess the impact of AAC volume on the BP and renal responses to RDN plus SSAHT and to SSAHT alone. Within each subgroup of patients defined according to tertile for AAC volume (tertiles 2 and 3 and tertile 1), we assessed the effect of treatment on BP parameters and eGFR by ANCOVA, including the baseline value as a covariable, as described previously.1 Between‐ and within‐observer reproducibility was assessed for AAC volume measurements by calculating intraclass correlation coefficients and their 95% confidence intervals (CIs).
Data are presented as mean±SD or median (interquartile range). Mean differences in BP and eGFR between baseline and 6 months are reported, with the 95% CI. We used SAS version 9.4 software (SAS Institute Inc). A P value <0.05 was considered significant.
Results
In total, 90 of the 106 patients randomized in the DENERHTN study took part in the CT substudy: 42 of 53 in the RDN group and 48 of 53 in the control group (Figure 2).
Figure 2.
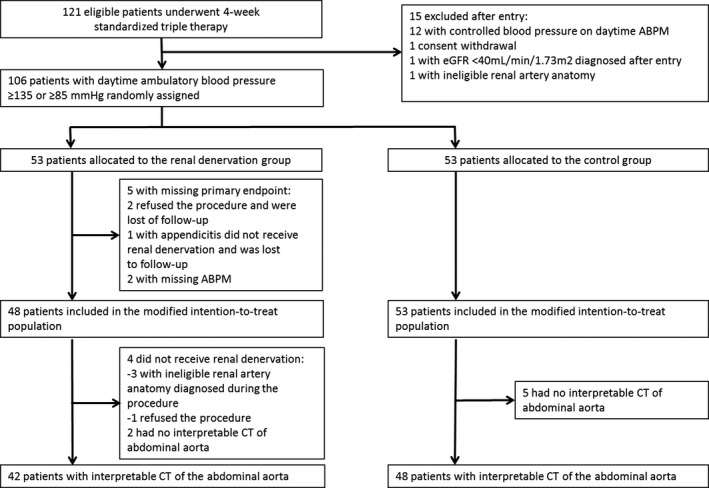
Flowchart of the study. No interpretable CT of the abdominal aorta indicates that the patients were evaluated at baseline by magnetic resonance imaging of the abdominal aorta, rather than CT angiogram. ABPM indicates ambulatory blood pressure monitoring; CT, computed tomography; eGFR, estimated glomerular filtration rate.
Clinical, Hemodynamic, and Biological Characteristics of the Patients at Baseline, by Tertile for AAC Volume
The within‐ and between‐observer intraclass correlation coefficients for AAC volume measurements were 0.96 (95% CI, 0.90–0.98) and 0.90 (95% CI, 0.75–0.96), respectively (P<0.001 for both). The range of AAC volume was 0 to 11.097 cm3 in the total patient population (n=90; tertile 1: 0–0.122 cm3; tertile 2: 0.123–1.466 cm3; tertile 3: 1.467–11.097 cm3). Nineteen of the 30 patients in tertile 1 (66%) had no visible AAC on CT scans.
The patients in the upper 2 tertiles for AAC volume were significantly older and more likely to smoke and to have type 2 diabetes mellitus, hyperlipidemia, ISH, and obstructive sleep apnea than the patients in tertile 1 (Table 1). They were also more likely to be male and white, with a history of cardiovascular events, including stroke, lower eGFR, and higher urinary albumin:creatinine ratio. The patients in the second and third tertiles for AAC volume had the highest daytime and 24‐hour ambulatory pulse pressure and PWV, with no difference between these 2 subgroups (Table 1). A similar but nonsignificant trend was observed for all other hemodynamic parameters.
Table 1.
Clinical and Biological Characteristics of Patients in the Entire Cohort and by Tertile for AAC Volume at Baseline
| First Tertile 0–122 mm3 | Second Tertile 123–1466 mm3 | Third Tertile 1467–11 097 mm3 | P Valuea | |
|---|---|---|---|---|
| n=30 | n=30 | n=30 | ||
| Clinical characteristics | ||||
| Age, y | 45.7±8.2b, c | 55.6±6.6c | 62.3±9.5 | <0.0001 |
| Male | 13 (43.3) | 21 (70.0) | 21 (70.0) | 0.0502 |
| White | 23 (76.7) | 19 (63.3) | 27 (90.0) | 0.0579 |
| Current or former smokers | 9 (30.0)c | 14 (46.7) | 23 (76.7) | 0.0012 |
| Type 2 diabetes mellitus | 0 (0.0)b, c | 9 (30.0) | 11 (36.7) | 0.0003 |
| Hyperlipidemia | 8 (26.7)c | 13 (43.3) | 18 (60.0) | 0.0336 |
| Prior cardiovascular event | 3 (10.0) | 8 (26.7) | 10 (33.3) | 0.0832 |
| Prior stroke | 0 (0.0) | 4 (13.3) | 4 (13.3) | 0.1195 |
| Obstructive sleep apnea | 5 (16.7) | 8 (26.7) | 14 (46.7) | 0.0422 |
| BMI, kg/m2 | 30.8±5.1 | 29.8±4.4 | 30.8±5.0 | 0.6616 |
| Biological characteristics | ||||
| Plasma creatinine, μmol/L | 77.4±26.9 | 85.7±19.5 | 86.9±23.6 | 0.2435 |
| eGFR, mL/min/1.73 m2 | 98.2±26.2 | 87.8±17.6 | 83.8±27.0 | 0.0643 |
| UACR, mg/mmold | 0.7 (0.5–1.8)c | 1.1 (0.4–4.0) | 3.0 (1.1–7.5) | 0.0264 |
| Hemodynamic parameters | ||||
| Office SBP, mm Hg | 151.4±20.7 | 157.7±19.7 | 162.0±22.3 | 0.1500 |
| Office DBP, mm Hg | 94.7±15.1 | 92.1±13.0 | 90.3±14.6 | 0.4784 |
| Office HR, beats/min | 72.9±10.3 | 71.0±11.8 | 73.4±11.7 | 0.7134 |
| Daytime ambulatory SBP, mm Hg | 150.4±17.2 | 154.4±15.4 | 155.7±17.0 | 0.4444 |
| Daytime ambulatory DBP, mm Hg | 95.8±12.5 | 91.4±13.9 | 91.3±13.4 | 0.3292 |
| Daytime ambulatory PP, mm Hg | 54.6±9.2b, c | 63.1±14.0 | 64.3±16.6 | 0.0140 |
| Nighttime ambulatory SBP, mm Hg | 136.2±16.5 | 140.6±17.2 | 140.7±15.7 | 0.4903 |
| Nighttime ambulatory DBP, mm Hg | 82.8±12.2 | 80.8±15.0 | 80.3±14.5 | 0.7682 |
| Nighttime ambulatory PP, mm Hg | 53.5±8.4 | 59.7±13.1 | 60.5±16.0 | 0.0747 |
| 24‐h ambulatory SBP, mm Hg | 146.8±16.7 | 150.5±15.9 | 151.6±16.2 | 0.4924 |
| 24‐h ambulatory DBP, mm Hg | 92.8±12.3 | 88.4±14.0 | 88.4±13.6 | 0.3451 |
| 24‐h ambulatory PP, mm Hg | 54.1±8.7c | 62.1±13.8 | 63.2±16.1 | 0.0171 |
| PWV, m/s | 9.7±2.2b, c | 11.6±2.8 | 12.8±2.8 | 0.0004 |
| Isolated systolic hypertension | 5 (16.7) | 9 (30.0) | 9 (30.0) | 0.4267 |
Data are expressed as mean±SD, n (%), or median (interquartile range). AAC indicates abdominal aortic calcification; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HR, heart rate; PP, pulse pressure; PWV, pulse wave velocity; SBP, systolic blood pressure; UACR, urinary albumin:creatinine ratio. Isolated systolic hypertension on ambulatory blood pressure monitoring was defined as daytime ambulatory SBP ≥135 mm Hg and DBP <85 mm Hg.
P values by ANOVA and Kruskal–Wallis tests for continuous variables and by χ2 and Fisher exact tests for categorical variables.
P<0.05 vs second tertile.
P<0.05 vs third tertile.
n=26, n=26, and n=24 for tertiles 1, 2 and 3, respectively (total: 76 measurements).
BP Response to RDN Plus SSAHT and SSAHT Alone at 6 Months by Tertile for AAC Volume
Regardless of tertile for AAC volume, there were no significant differences in baseline characteristics between the RDN and control groups (Table 2), and these characteristics were similar to those previously reported for the DENERHTN study.1 The median volume of AAC did not significantly differ between the RDN and control groups, but there was considerable between‐patient variability in AAC volume (0.448 cm3 [interquartile range: 0.098–2.055 cm3] versus 0.433 cm3 [interquartile range: 0.012–2.310 cm3], respectively; Table 2). We previously showed that at 6 months follow‐up, RDN combined with a median number of 5 drugs of the SSAHT significantly reduced daytime (primary end point), nighttime, and 24‐hour ambulatory SBP by ≈6 mm Hg more than with 5 drugs of the SSAHT alone, regardless of AAC volume tertile.1 The proportion of patients with full adherence to the SSAHT at 6 months did not differ significantly between the 2 groups (51.3% for RDN group versus : 50.0% for control group; P=0.9093).
Table 2.
Baseline Comparison of the RDN and Control Groups in the CT Angiogram Substudy
| All | RDN | Control | P Valuea | |
|---|---|---|---|---|
| n=90 | n=42 | n=48 | ||
| Clinical characteristics | ||||
| Age, y | 54.6±10.6 | 54.5±11.1 | 54.6±10.3 | 0.9817 |
| Male | 55 (61.1) | 26 (61.9) | 29 (60.4) | 0.8851 |
| White | 69 (76.7) | 33 (78.6) | 36 (75.0) | 0.6894 |
| Nonsmokers | 44 (48.9) | 20 (47.6) | 24 (50.0) | 0.3373 |
| Type 2 diabetes mellitus | 20 (22.2) | 7 (16.7) | 13 (27.1) | 0.2357 |
| Hyperlipidemia | 39 (43.3) | 19 (45.2) | 20 (41.7) | 0.7330 |
| Prior cardiovascular event | 21 (23.3) | 13 (31.0) | 8 (16.7) | 0.1099 |
| Prior stoke | 8 (8.9) | 5 (11.9) | 3 (6.3) | 0.4654 |
| Obstructive sleep apnea | 27 (30.0) | 14 (33.3) | 13 (27.1) | 0.5186 |
| BMI, kg/m2 | 30.5±4.8 | 30.8±5.2 | 30.2±4.4 | 0.5307 |
| Biological characteristics | ||||
| Plasma creatinine, μmol/L | 83.4±23.6 | 83.2±22.7 | 83.6±24.6 | 0.9481 |
| eGFR, mL/min/1.73 m2 | 89.8±24.5 | 89.9±25.2 | 89.8±24.1 | 0.9845 |
| Cardiovascular parameters | ||||
| Office SBP, mm Hg | 157.0±21.1 | 159.0±22.1 | 155.3±20.3 | 0.4023 |
| Office DBP, mm Hg | 92.3±14.2 | 94.0±15.1 | 90.9±13.4 | 0.3140 |
| Office HR, beats/min | 72.4±11.2 | 71.5±10.6 | 73.2±11.8 | 0.4691 |
| Daytime ambulatory SBP, mm Hg | 153.5±16.5 | 155.9±16.7 | 151.4±16.2 | 0.1960 |
| Daytime ambulatory DBP, mm Hg | 92.8±13.3 | 93.5±15.6 | 92.3±11.1 | 0.6816 |
| Daytime ambulatory PP, mm Hg | 60.7±14.2 | 62.5±14.3 | 59.1±14.0 | 0.2619 |
| Nighttime ambulatory SBP, mm Hg | 139.2±16.4 | 142.8±17.7 | 136.0±14.7 | 0.0478 |
| Nighttime ambulatory DBP, mm Hg | 81.3±13.8 | 83.4±16.4 | 79.4±10.9 | 0.1715 |
| Nighttime ambulatory PP, mm Hg | 57.9±13.1 | 59.4±12.9 | 56.6±13.4 | 0.3082 |
| 24‐h ambulatory SBP, mm Hg | 149.7±16.2 | 152.4±16.9 | 147.3±15.4 | 0.1400 |
| 24‐h ambulatory DBP, mm Hg | 89.9±13.3 | 90.8±15.7 | 89.0±10.9 | 0.5178 |
| 24‐h ambulatory PP, mm Hg | 59.8±13.7 | 61.5±13.7 | 58.3±13.7 | 0.2675 |
| PWV, m/s | 11.3±2.9 | 11.7±2.8 | 11.0±3.0 | 0.2964 |
| AAC, cm3 | ||||
| All | 0.448 (0.098–2.055) | 0.433 (0.012–2.310) | 0.7954 | |
| Tertile 1 | 30 (33.3) | 13 (31.0) | 17 (35.4) | 0.3916 |
| Tertile 2 | 30 (33.3) | 17 (40.5) | 13 (27.1) | |
| Tertile 3 | 30 (33.3) | 12 (28.6) | 18 (37.5) | |
Data are expressed as mean±SD, n (%), or median (interquartile range). AAC indicates abdominal aortic calcifications; BMI, body mass index; CT, computed tomography; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HR, heart rate; PP, pulse pressure; PWV, pulse wave velocity; RDN, renal denervation; SBP, systolic blood pressure.
P values were obtained by unpaired t tests and Mann–Whitney U tests for continuous variables and by χ2 and Fisher exact tests for discrete variables.
Two contrasting patterns were observed in the analysis of BP response by AAC volume tertile. Indeed, the baseline‐adjusted difference in the change in daytime ambulatory SBP from baseline to 6 months between the RDN and control groups was −10.1 mm Hg (95% CI, −20.0 to −0.2 mm Hg, P=0.0462) for the patients in tertile 1 but only −2.5 mm Hg (95% CI, −9.9 to 4.9 mm Hg, P=0.4987) for the patients in combined tertiles 2 and 3 (Table 3). A similar but nonsignificant trend was observed for the baseline‐adjusted difference in the change in nighttime and 24‐hour ambulatory SBP according to AAC volume tertile (Table 3). Changes in daytime ambulatory SBP from baseline are shown in Figure 3. We observed no significant difference in the change from baseline to 6 months for ambulatory diastolic BP and heart rate between the RDN and control groups, according to AAC volume tertile (Tables 4 and 5).
Table 3.
ASBP at Randomization and After 6‐Mo Follow‐up by Tertile for AAC Volume in the RDN and Control Groups
| RDN Group | Control Group | Mean Baseline‐Adjusted Difference (95% CI) Between the 2 Groups at 6 Moa | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Randomization (Mean±SD) | 6 Mo (Mean±SD) | Mean Baseline‐Adjusted Difference (95% CI) | Randomization (Mean±SD) | 6 Mo (Mean±SD) | Mean Baseline‐Adjusted Difference (95% CI) | |||
| Tertile 1 | ||||||||
| ASBP, mm Hg | (n=13) | (n=13) | (n=17) | (n=17) | ||||
| Daytime | 154.0±17.5 | 135.1±12.6 | −17.6 (−25.0 to −10.2) | 147.7±16.9 | 141.2±19.2 | −7.5 (−14.0 to −1.1) | −10.1 (−20.0 to −0.2) | 0.0462 |
| Nighttime | 142.8±18.8 | 126.4±11.0 | −13.5 (−21.8 to −5.2) | 131.2±12.8 | 122.9±19.4 | −10.5 (−17.7 to −3.3) | −3.1 (−14.4 to 8.3) | 0.5828 |
| 24‐h | 151.2±17.9 | 132.8±11.8 | −16.7 (−24 to −9.3) | 143.5±15.4 | 136.6±18.8 | −8.2 (−14.6 to −1.8) | −8.5 (−18.4 to 1.5) | 0.0921 |
| Tertiles 2 and 3 | ||||||||
| ASBP, mm Hg | (n=29) | (n=29) | (n=31) | (n=31) | ||||
| Daytime | 156.8±16.6 | 141.9±20.5 | −14.5 (−19.8 to −9.2) | 153.4±15.7 | 141.8±17.0 | −12 (−17.1 to −6.8) | −2.5 (−9.9 to 4.9) | 0.4987 |
| Nighttime | 142.9±17.6 | 128.8±21.8 | −13.7 (−19.2 to −8.2) | 138.6±15.1 | 132.4±17.4 | −6.6 (−12.0 to −1.3) | −7.0 (−14.7 to 0.7) | 0.0729 |
| 24‐h | 152.9±16.7 | 137.9±20.6 | −14.6 (−19.6 to −9.6) | 149.4±15.3 | 139.0±15.9 | −10.7 (−15.6 to −5.9) | −3.8 (−10.8 to 3.1) | 0.2730 |
AAC indicates abdominal aortic calcifications; ASBP, ambulatory systolic blood pressure; CI, confidence interval; RDN, renal denervation.
RDN group vs control group.
Figure 3.
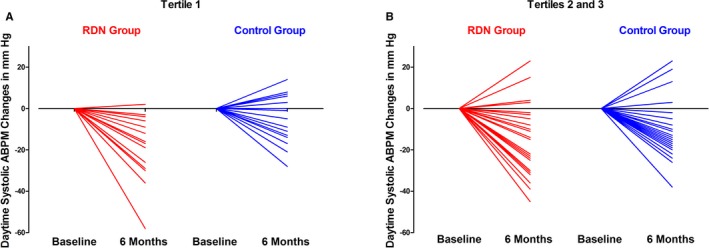
Individual changes in daytime ABPM, by tertile, for abdominal aortic calcification in the RDN group and in the control group, after 6 months of follow‐up (A: tertile 1; B: tertiles 2 and 3). AAC indicates abdominal aortic calcifications; ABPM, ambulatory blood pressure monitoring; RDN, renal denervation.
Table 4.
ADBP at Randomization and After 6‐Mo Follow‐up, by Tertile for AAC Volume
| RDN Group | Control Group | Mean Baseline‐Adjusted Difference (95% CI) Between the 2 Groups at 6 Moa | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Randomization (Mean±SD) | 6 Mo (Mean±SD) | Mean Baseline‐Adjusted Difference (95% CI) | Randomization (Mean±SD) | 6 Mo (Mean±SD) | Mean Baseline‐Adjusted Difference (95% CI) | |||
| Tertile 1 | ||||||||
| ADBP, mm Hg | (n=13) | (n=13) | (n=17) | (n=17) | ||||
| Daytime | 99.8±14.7 | 86.7±11.9 | −11.3 (−17.3 to −5.4) | 92.8±10.0 | 88.8±12.3 | −5.3 (−10.4 to −0.1) | −6.1 (−14.1 to 2.0) | 0.1325 |
| Nighttime | 89.6±14.3 | 78.0±11.7 | −8.6 (−14.7 to −2.4) | 77.5±7.2 | 73.1±11.3 | −6.7 (−12.0 to −1.4) | −1.8 (−10.5 to 6.9) | 0.6708 |
| 24‐h | 97.4±14.6 | 84.5±11.6 | −10.9 (−16.6 to −5.2) | 89.2±9.1 | 84.9±11.6 | −5.8 (−10.8 to −0.9) | −5.1 (−12.8 to 2.7) | 0.1942 |
| Tertiles 2 and 3 | ||||||||
| ADBP, mm Hg | (n=29) | (n=29) | (n=31) | (n=31) | ||||
| Daytime | 90.6±15.3 | 82.2±14.7 | −8.5 (−11.7 to −5.3) | 92.0±11.8 | 83.4±13.8 | −8.5 (−11.7 to −5.4) | 0.0 (−4.5 to 4.5) | 0.9885 |
| Nighttime | 80.7±16.8 | 72.9±13.7 | −7.7 (−10.5 to −4.9) | 80.5±12.5 | 75.4±12.3 | −5.1 (−7.8 to −2.4) | −2.6 (−6.5 to 1.3) | 0.1811 |
| 24‐h | 87.9±15.5 | 79.3±13.7 | −8.7 (−11.4 to −5.9) | 88.9±11.9 | 81.0±12.6 | −7.7 (−10.4 to −5.1) | −0.9 (−4.7 to 2.9) | 0.6346 |
AAC indicates abdominal aortic calcifications; ADBP, ambulatory diastolic blood pressure; CI, confidence interval; RDN, renal denervation.
RDN group vs control group.
Table 5.
Ambulatory Heart Rate at Randomization and After 6‐Mo Follow‐up by Tertile for AAC Volume in the RDN and Control Groups
| RDN | Control Group | Mean Baseline‐Adjusted Difference (95% CI) Between the 2 Groups at 6 Moa | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Randomization (Mean±SD) | 6 Mo (Mean±SD) | Mean Baseline‐Adjusted Difference (95% CI) | Randomization (Mean±SD) | 6 Mo (Mean±SD) | Mean Baseline‐Adjusted Difference (95% CI) | |||
| Tertile 1 | ||||||||
| HR, beats/min | (n=13) | (n=13) | (n=17) | (n=17) | ||||
| Daytime | 79.2±10.7 | 74.5±9.8 | −5.4 (−9.9 to −0.9) | 82.0±8.6 | 72.2±8.9 | −9.3 (−13.2 to −5.3) | 3.8 (−2.2 to 9.9) | 0.2024 |
| Nighttime | 68.8±7.7 | 67.2±8.1 | −1.1 (−4.6 to 2.4) | 66.8±7.8 | 62.6±6.6 | −4.6 (−7.7 to −1.6) | 3.5 (−1.1 to 8.2) | 0.1288 |
| 24‐h | 76.8±10.1 | 72.5±9.2 | −4.6 (−8.6 to −0.7) | 78.4±7.8 | 69.6±7.7 | −8.4 (−11.9 to −5.0) | 3.8 (−1.4 to 9.0) | 0.1460 |
| Tertiles 2 and 3 | ||||||||
| HR, beats/min | (n=29) | (n=29) | (n=31) | (n=31) | ||||
| Daytime | 74.7±11.7 | 66.5±10.4 | −8.7 (−12.6 to −4.8) | 77.2±9.7 | 68.8±13.8 | −7.9 (−11.7 to −4.1) | −0.8 (−6.2 to 4.7) | 0.7770 |
| Nighttime | 68.0±10.6 | 61.6±10.2 | −6.3 (−9.6 to −3.0) | 66.9±8.0 | 62.5±11.9 | −4.5 (−7.7 to −1.3) | −1.8 (−6.4 to 2.8) | 0.4402 |
| 24‐h | 72.9±11.4 | 65.1±10.1 | −8.1 (−11.7 to −4.5) | 74.5±9.2 | 67.0±12.8 | −7.2 (−10.7 to −3.7) | −0.9 (−5.9 to 4.1) | 0.7318 |
AAC indicates abdominal aortic calcifications; CI, confidence interval; HR, heart rate; RDN, renal denervation.
RDN group vs control group.
The number of antihypertensive drugs prescribed at 6 months did not differ significantly between the RDN and control groups for any of the AAC volume tertiles (Figure 4). The proportion of patients with full adherence to SSAHT at 6 months did not differ significantly between the RDN and control groups for any AAC volume tertile (Figure 4). The total number of ablations during the RDN procedure did not differ between tertiles for AAC volume (tertiles 2 and 3: 11.0 [10.0–12.0]; tertile 1: 11.0 [10.0–13.0]; P=0.8371).
Figure 4.
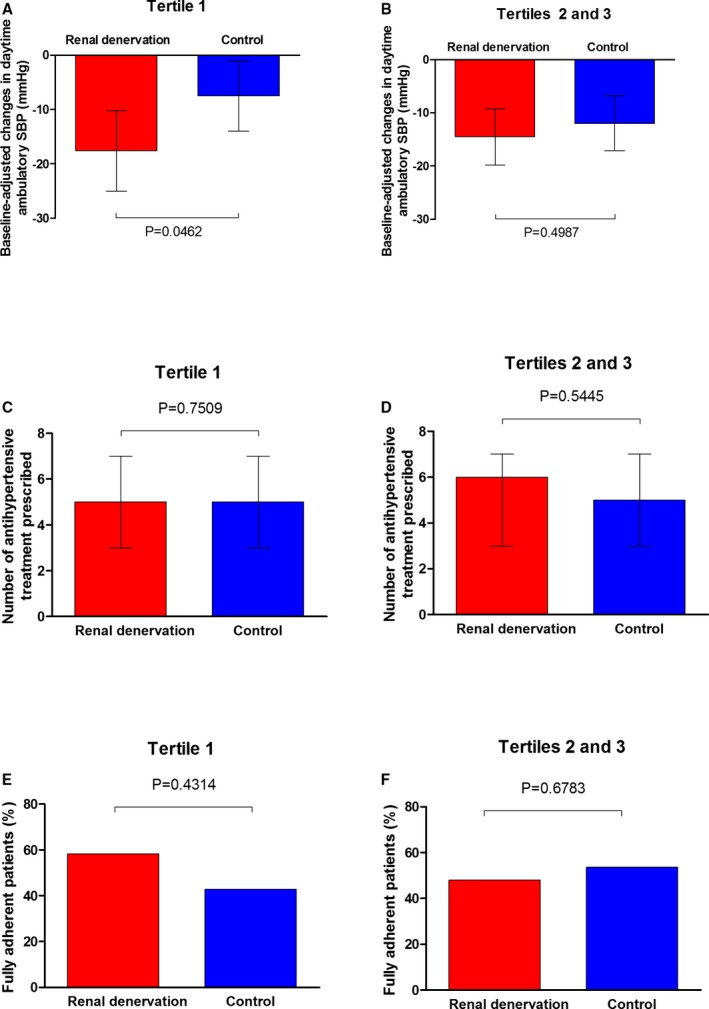
Baseline‐adjusted changes in daytime ambulatory systolic blood pressure (A and B), number of antihypertensive treatments prescribed (C and D) and percentage of fully adherent patients (E and F) in the renal denervation (red) and control (blue) groups by tertile (tertile 1 vs tertiles 2 and 3 for abdominal aortic calcification volume).
Relationship Between the BP Response to RDN Plus SSAHT and SSAHT and eGFR Changes, by Tertile for AAC Volume
Regardless of tertile for AAC volume, we previously reported a similar decrease in eGFR in both the RDN and control groups by about 5 mL/min per 1.73 m2 at 6 months follow‐up.1
However, the analysis of the renal response to RDN by AAC volume tertile revealed 2 contrasting patterns for the BP response. Indeed, eGFR remained stable in the patients in tertile 1 who underwent RDN but decreased significantly in the other 3 subgroups (Table 6). The baseline‐adjusted difference in the change in eGFR from baseline to 6 months between the RDN and control groups was +10.5 mL/min per 1.73 m2 (95% CI, +2.2 to +18.8; P=0.0148) in the patients in tertile 1 and −3.8 mL/min per 1.73 m2 (95% CI, −10.6 to 3; P=0.2640) in the patients in combined tertiles 2 and 3 (Table 6).
Table 6.
eGFR at Randomization and After 6‐Mo Follow‐up by Tertile for AAC Volume in the RDN and Control Groups
| RDN Group | Control Group | Mean Baseline‐Adjusted Difference (95% CI) Between the 2 Groups at 6 Moa | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Randomization (Mean±SD) | 6 Mo (Mean±SD) | Mean Baseline‐Adjusted Difference (95% CI) | Randomization (Mean±SD) | 6 Mo (Mean±SD) | Mean Baseline‐Adjusted Difference (95% CI) | |||
| Tertile 1 | ||||||||
| eGFR, mL/min | (n=12) | (n=13) | (n=17) | (n=17) | ||||
| 99.1±31.9 | 100.0±28.5 | 2.5 (−3.8 to 8.8) | 97.6±22.4 | 89.6±20.9 | −8.0 (−13.4 to −2.7) | 10.5 (2.2–18.8) | 0.0148 | |
| Tertiles 2 and 3 | ||||||||
| eGFR, mL/min | (n=29) | (n=27) | (n=31) | (n=31) | ||||
| 86.1±21.3 | 76.8±21.9 | −7.5 (−12.5 to −2.6) | 85.5±24.3 | 81.7±23.5 | −3.7 (−8.3 to 0.9) | −3.8 (−10.6 to 3.0) | 0.2640 | |
AAC indicates abdominal aortic calcifications; CI, confidence interval; eGFR, estimated glomerular filtration rate; RDN, renal denervation.
RDN group vs control group.
We plotted eGFR changes in response to the BP changes by AAC volume tertile for the RDN and control groups (Figure 5). The RDN‐induced decrease in daytime ambulatory SBP, which was greatest in the patients of tertile 1, was associated with no change in eGFR at 6 months (Figure 5). Conversely, the decrease in daytime ambulatory SBP was associated with a decrease in eGFR in the other subgroups (Figure 5).
Figure 5.
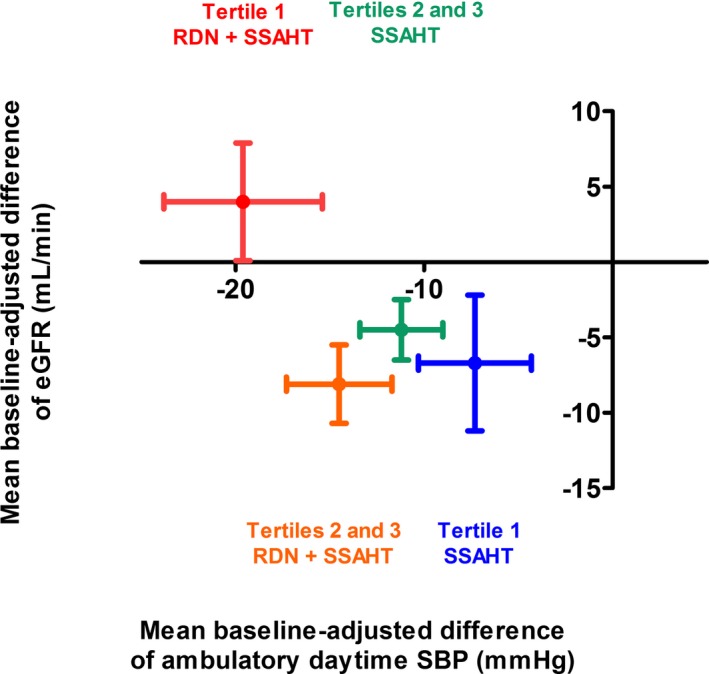
Relationship between the changes from baseline to 6 months in mean baseline‐adjusted eGFR and daytime ambulatory SBP in the 4 subgroups of patients. Red plot: patients of tertile 1 for AAC volume treated with RDN plus SSAHT; green plot: patients of tertiles 2 and 3 for AAC volume treated with RDN plus SSAHT; blue plot: patients of tertile 1 for AAC volume treated with SSAHT alone; orange plot: patients of tertiles 2 and 3 for AAC volume treated with SSAHT alone. The data shown are mean±SD. AAC indicates abdominal aortic calcifications; eGFR, estimated glomerular filtration rate; RDN, renal denervation; SBP, systolic blood pressure; SSAHT, standardized stepped‐care antihypertensive treatment.
There was no significant change in urinary albumin:creatinine ratio during follow‐up, and the difference between groups and within each stratum of AAC volume was not significant (not shown).
Discussion
This post hoc exploratory analysis of the DENERHTN trial investigated the impact of AAC burden, as assessed by pre‐RDN abdominal CT scans, on 6‐month ambulatory BP and eGFR responses to RDN and SSAHT in patients with resistant hypertension. Our results show that the combination of RDN and SSAHT reduced 6‐month daytime ambulatory SBP significantly more strongly (by ≈10 mm Hg) than the same SSAHT alone in the patients with the lowest AAC volumes (tertile 1, range: 0–0.122 cm3). In contrast, the difference in the change in daytime ambulatory SBP between the RDN and control groups was much smaller (≈2–3 mm Hg) and not significant in patients with greater atherosclerotic burden (tertiles 2 and 3 for AAC volume). The change in eGFR from baseline to 6 months in response to RDN was also influenced by AAC burden. Indeed, eGFR remained stable in the patients in tertile 1 who underwent RDN (+2.5 mL/min per 1.73 m2) but decreased in the other 3 subgroups (by ≈4–8 mL/min per 1.73 m2). These preliminary results suggest that measurement of AAC burden on abdominal CT scans without contrast staining before RDN may be a new predictor of short‐term BP and renal responses to RDN.
We report the first quantification of AAC burden in a population of patients with resistant hypertension selected for the DENERHTN trial. AAC has been studied extensively in other clinical settings, including patients with high cardiovascular risk or chronic kidney disease,15, 16 and the extent of these calcifications has been associated with a poor cardiovascular and renal prognosis.14, 15 The radiological methods used in these studies included qualitative or semiquantitative measurements of AAC burden on lateral lumbar x‐rays, aortic angiograms, and CT scans.17, 18, 19 In the DENERHTN study, we performed an abdominal CT scan (or MRI in a few cases, n=7) for patients with resistant hypertension before randomization to exclude secondary hypertension and to assess the suitability of the renal artery anatomy for RDN. All CT scans were sent to the imaging core laboratory, where the volume of AAC was measured on noncontrast images, with semiautomatic software, with good within‐ and between‐observer reproducibility, as reported in other studies.7, 19 About 80% of our patients with resistant hypertension confirmed by ambulatory BP monitoring had visible AAC. Median AAC volume was 0.438 cm3, but there were considerable differences between patients (0–11.100 cm3). This prevalence of AAC is higher than that reported in a large, independently living, middle‐aged, and mostly asymptomatic US population of 6456 participants (28% with hypertension) in whom the prevalence of AAC was 54.3% on noncontrast CT imaging.20 Our results also expand those of a small study reporting AAC volume on CT scan to be greater in 15 patients with treatment‐resistant hypertension or with ISH than in normotensive participants.6, 7 The baseline clinical and biological characteristics of our patients with resistant hypertension and the highest AAC burden were consistent with previous reports from normotensive and hypertensive cohorts.7 These patients tended to be older, with a history of smoking, type 2 diabetes mellitus, hyperlipidemia, obstructive sleep apnea, and prior cardiovascular events, and they were more likely to have a lower eGFR. As expected, higher AAC volume was also associated with greater arterial stiffness, as shown by the higher carotid–femoral PWV and ambulatory pulse pressure and, consequently, a higher proportion of patients with ISH in tertiles 2 and 3 than in tertile 1 at baseline. The pathophysiological mechanisms underlying the development of arterial calcifications involving the abdominal aorta are complex and multifactorial.16
The principal finding of our post hoc analysis was that AAC burden influences both the systemic and renal hemodynamic responses to RDN at 6 months, regardless of the number of antihypertensive drugs used or adherence to the SSAHT, which were similar for all AAC tertiles. Indeed, the largest significant difference in the change in daytime ambulatory SBP in favor of RDN (−10 mm Hg) was observed in the patients in tertile 1, those with the lowest atherosclerotic burden. Conversely, RDN was less effective at lowering BP relative to the control group (−2.5 mm Hg) in patients with the highest AAC burden (tertiles 2 and 3). The apparently greater ambulatory SBP response to the SSAHT alone in the patients in combined tertiles 2 and 3 than in those of tertile 1 can be explained, in part, by the higher ambulatory SBP of these patients at baseline (see Table 3). The weaker BP response to RDN in the patients with the greatest AAC burden is consistent with the lower office and ambulatory BP reported in patients with ISH than in those with both systolic and diastolic hypertension.9, 10 However, despite the higher frequency of ISH in the patients in tertiles 2 and 3 for AAC volume than in those in tertile 1, these 2 conditions were not strictly superimposable. Indeed, 20% of patients with ISH had no visible AAC, and ≈70% of the patients with the highest AAC burden (tertiles 2 and 3) had combined systolic and diastolic hypertension without ISH; only 30% of the patients had both conditions (ISH and a high AAC burden). The presence of AAC is a BP‐independent marker of aortic stiffness and thus of vascular aging7 that also takes into account the overall extent of atherosclerosis burden and is associated with a risk of poor cardiovascular outcome.11 In our study, high AAC volume was an integrative biomarker of the multiple factors associated with lower efficacy of RDN for lowering BP. These factors included older age,21 ISH,9, 10 lower GFR,21 and vascular stiffness.22 Conversely, the larger BP response to RDN than to SSAHT in patients with little or no AAC, and thus less vascular remodeling, may reflect the predominant contribution of the renal sympathetic drive to the pathophysiology of resistant hypertension in these patients, who tend to be younger and to have fewer comorbid conditions, consistent with the results of renal norepinephrine spillover experiments.23
The relationship between daytime ambulatory SBP decrease and the change in eGFR at 6 months also differed between the AAC volume tertile subgroups. Indeed, eGFR was found to remain stable 6 months after RDN in the patients in tertile 1, who displayed the largest decrease in BP. Conversely, eGFR decreased in parallel with BP in the other 3 subgroups. A small decrease in eGFR is usually observed in the context of better BP control in patients with hypertension, due to intraglomerular pressure reduction.24 In cases of long‐standing hypertension, microvascular disease is observed in the kidney, characterized by a blunted vasoconstrictive or vasodilatory capacity of the preglomerular afferent arterioles in response to changes in renal perfusion pressure; therefore, any change in systemic and renal pressure is associated with a proportional change in GFR.8 This phenomenon is exacerbated in the presence of AAC and aortic stiffening, which induce the transmission of abnormally high pulsatile stresses in renal microvessels.25, 26 In this pathophysiological context, the maintenance of eGFR despite a large decrease in BP suggests that the addition of RDN to SSAHT including a renin–angiotensin system blocker and a calcium channel blocker (acting on postglomerular and both pre‐and postglomerular resistances,27 respectively) may have induced specific and subtle renal hemodynamic changes detectable only in the patients with the lowest AAC burden, as expected from experimental models.28, 29 A small cohort study reported an increase in renal artery mean flow 6 months after RDN, as assessed by 3‐dimensional magnetic resonance angiography.30 In addition, renal vascular resistance has been shown to decrease 3 to 6 months after RDN in patients with resistant hypertension,31, 32 with no significant increase in renal perfusion detectable on MRI33 or improvement in renal oxygenation, as determined by blood oxygen level–dependent MRI.32 However, in a small cohort study of 27 patients with stage 3 or 4 chronic kidney disease, the annual decrease in eGFR was slightly smaller after than before RDN, and this effect was accompanied by a significant decrease in office BP.34 These mixed renal effects of RDN may be caused by (1) incomplete RDN, (2) the absence of a control group, or (3) the heterogeneity of the patients, because none of the studies checked for an influence of AAC burden, even though they included patients with chronic kidney disease.
Finally, our results suggest that changes in the viscoelastic properties of the aorta, and likely of large arteries in other territories, associated with increasing AAC volume are probably contributing factors influencing variability in the BP and renal responses to RDN. The other factors identified include high BP at baseline, older age, being white, preserved renal function, previous use of spironolactone, adherence to antihypertensive treatment, and the presence of ISH and of accessory renal arteries.1, 2, 9, 10, 21, 35, 36 The identification of predictors of BP and renal responses to RDN, for use before and during the RDN procedure, remains crucial.5
Study Limitations
The limitations of the DENERHTN trial have been discussed elsewhere.1 The limitations of this post hoc analysis include its exploratory nature, allowing only hypothesis‐generating conclusions to be drawn. Other limitations include the following: (1) The study was not designed to explore the underlying pathophysiologic mechanisms of the differential hemodynamic and renal effects of RDN according to atherosclerotic burden, and thus GFR and renal plasma flow were not measured with exogenous markers; (2) none of the patients had baseline eGFR values <40 mL/min per 1.73 m2; (3) we cannot fully exclude a “Hawthorne” effect, because there was no sham group.37 However, the differential BP and renal responses to RDN 6 months after baseline, according to AAC volume tertile, were not confounded by differences in the number of renal ablations in the RDN groups, the number of antihypertensive drugs prescribed at 6 months in any of the subgroups, or adherence to SSAHT at 6 months, as assessed by drug detection in the urine or plasma in any of the subgroups. However, the technical success of renal nerve ablation with the Symplicity catheter might vary, resulting in variable degrees of renal nerve damage that can be an independent contributor to the BP response. The large differences in BP and renal responses to RDN between tertiles for AAC volume suggest (1) that AAC burden influences the BP and renal responses to RDN and (2) that their measurements on pre‐RDN noncontrast abdominal CT scan should be included in ongoing clinical trials. However, our thresholds of AAC volume are not directly applicable to other populations of patients because they rely on the CT scan, the image characteristics, and the software used to detect and measure AAC. Consequently, each center should determine its thresholds of AAC volume.
Conclusion
This post hoc exploratory analysis of the DENERHTN trial taking into account the AAC burden, as assessed by a semiautomatic method on pre‐RDN noncontrast abdominal CT scans, suggested differential patterns of systemic and renal hemodynamic response to RDN in the short term.
We found that the additional reduction in daytime ambulatory SBP achieved by adding RDN to SSAHT (1) was largest (≈10 mm Hg) and statistically significant in patients with the lowest atherosclerotic burden and (2) was not associated with a decrease in eGFR. Further studies will be required to confirm these preliminary results and to determine whether the short‐term renal hemodynamic effects of RDN in patients with a low atherosclerotic burden have a beneficial effect in the long term. A loss in eGFR of 9.3 mL/min per 1.73 m2 at 3 years was observed in the SYMPLICITY HTN‐1 cohort study, but this study did not take into account the AAC burden.38
Because RDN appears to be associated with a limited risk of adverse events,1, 35 randomized sham‐controlled trials are currently under way to assess the efficacy of RDN for lowering ambulatory BP and its safety, with various radiofrequency‐ or ultrasound‐based denervation catheters or externally delivered ultrasound technologies; the preliminary results of these studies are expected in 2018.5 These trials include patients with resistant hypertension treated with standardized antihypertensive treatment regimens and with strict monitoring of adherence to treatment. However, they also include patients with milder forms of hypertension whose treatment with antihypertensive drugs could be stopped for a limited period.39 Our results suggest that the evaluation of AAC burden on pre‐RDN noncontrast CT scans should be part of the data collected during the trials to analyze the BP and eGFR results taking into account the AAC burden.
Appendix
List of the DENERHTN Investigators
The following investigators (with the number of patients enrolled and randomized at each center given in parentheses) and committees participated in the DENERHTN trial: Hôpital Européen Georges Pompidou, Paris (31/28)—L. Amar, G. Bobrie, A. Lorthioir, M. Monge, J.‐Y. Pagny, P. F. Plouin, M. Sapoval; Hôpital Cardiologique, Lille (20/15)—G. Claisse, P. Delsart, M. Midulla, C. Mounier‐Vehier; Hôpital de la Croix Rousse and Hôpital Edouard Herriot, Lyon (14/13)—P. Y. Courand, R. Dauphin, J. P. Fauvel, P. Lantelme, O. Rouvière; Hôpital Saint André and Hôpital Pellegrin, Bordeaux (14/13)—A. Cremer, P. Gosse, N. Grenier, Y. Lebras, H. Trillaud; Hôpital Arthur Gardiner, Dinard and CHU Rennes (12/12)—T. Denolle, C. Dourmap‐Collas, J. F. Heautot, A. Larralde, F. Paillard; Hôpital de la Pitié Salpétrière, Paris (6/5)—P. Cluzel, X. Girerd, D. Rosenbaum; Hôpital Bretonneau, Tours (5/4)—D. Alison, J. M. Halimi; CHU Nancy‐Brabois, Nancy (4/1)—M. Claudon, B. Popovic, P. Rossignol, F. Zannad; CHU de Grenoble, Grenoble (3/3)—J. P. Baguet, O. Ormezzano, F. Thony; CHU de la Timone, Marseille (3/3)—J. M. Bartoli, B. Vaïsse; Hôpital la Milétrie, Poitiers (3/3)—J. Drouineau, D. Herpin, P. Sosner, J. P. Tasu, S. Velasco; Hôpital Lapeyronie and Hôpital Arnaud de Villeneuve, Montpellier (2/2)—J. Ribstein, H. Vernhet‐Kovacsik; CHU Rangueil, Toulouse (2/2)—B. Bouhanick, B. Chamontin, H. Rousseau; Hôpital Avicenne, Bobigny (1/1)—S. Le Jeune, M. Lopez‐Sublet, J. J. Mourad; Hôpital Pasteur, Nice (1/1)—L. Bellmann, V. Esnault, E. Ferrari; Scientific Committee—M. Azizi (Chair), M. Sapoval (Co‐Chair), G. Bobrie, G. Chatellier, P. F. Plouin, F. Zannad, J. M. Halimi, J. P. Baguet, H. Vernhet‐Kovacsik, I. Durand‐Zaleski; Data Safety Committee—J. P. Beregi (Chair), M. Lièvre, A. Persu.
Sources of Funding
This trial was sponsored by the Assistance Publique‐Hôpitaux de Paris and was funded by a grant from the French Ministry of Health (Soutien aux Technologies Innovantes et Coûteuses 2011, IC110175).
Disclosures
Bobrie received grant from the French Health Minister. Delsart has no conflict of interest. Claire Mounier‐Véhier has received personal fees from Bayer Pharma and Non‐financial support from Bayer Pharma, Novartis, MSD, Daichi Sankyo, Astra Zénéca, Leo Pharma and Novonordisk. Girerd received personal fees form Bouchara Recodati, Sanofi, Merck and Sothema. Rossignol received personal fees from Novartis, Relypsa, Astra‐Zeneca, Stealth Peptides, CVRx, Fresenius, Vifor Fresenius Medical Care Renal Pharma, CTMA and is cofounder of CardioRenal. Zannad received personal fees from Janssen, Bayer, Pfizer, Novartis, Boston Scientific, Resmed, Amgen, CVRx, Quantum Genomics, Takeda, General Electric, Boehringer, Relypsa, Zs Pharma, Astra Zeneca, Roche Diagnostics and was founder of cardiorenal and CVCT. Ribstein received grants from Otsuka and consulting fees from Bayer, Bristol‐Myers‐Squibb, Menarini, Novartis, Sanofi‐Aventis and Servier. Azizi has received honoraria for advisory board meetings from Vessix, Boston Scientific Corporation, Cordis, Actelion, has received speakers’ honoraria from Cordis, CVRx, Servier; was involved as investigator in Symplicity HTN‐2 (Ardian/Medtronic) and Reduce‐HTN (Vessix/Boston Scientific Corporation) trials; has received a research grant from Servier, Novartis, Recor, Quantum genomics. The remaining authors have no disclosures to report.
Acknowledgments
We thank Pascaline Aucouturier, Nicolas Hollebecq, Nathalie Gomes, Laroussi Ben Zrig, and Abderraouf Bensaber from the Clinical Trials Unit of Georges Pompidou European Hospital for organizing the trial, and for data monitoring, collection, and management. We thank all the nursing teams and the DENERHTN investigators for their involvement in patient care.
(J Am Heart Assoc. 2017;6:e007062 DOI: 10.1161/JAHA.117.007062.)
References
- 1. Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, Midulla M, Mounier‐Vehier C, Courand PY, Lantelme P, Denolle T, Dourmap‐Collas C, Trillaud H, Pereira H, Plouin PF, Chatellier G. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open‐label, randomised controlled trial. Lancet. 2015;385:1957–1965. [DOI] [PubMed] [Google Scholar]
- 2. Azizi M, Pereira H, Hamdidouche I, Gosse P, Monge M, Bobrie G, Delsart P, Mounier‐Vehier C, Courand PY, Lantelme P, Denolle T, Dourmap‐Collas C, Girerd X, Halimi JM, Zannad F, Ormezzano O, Vaisse B, Herpin D, Ribstein J, Chamontin B, Mourad JJ, Ferrari E, Plouin PF, Jullien V, Sapoval M, Chatellier G. Adherence to antihypertensive treatment and the blood pressure lowering effects of renal denervation in the renal denervation for hypertension (DENERHTN) trial. Circulation. 2016;134:847–857. [DOI] [PubMed] [Google Scholar]
- 3. Gosse P, Cremer A, Pereira H, Bobrie G, Chatellier G, Chamontin B, Courand PY, Delsart P, Denolle T, Dourmap C, Ferrari E, Girerd X, Halimi JM, Herpin D, Lantelme P, Monge M, Mounier‐Vehier C, Mourad JJ, Ormezzano O, Ribstein J, Rossignol P, Sapoval M, Vaïsse B, Zannad F, Azizi M. Twenty‐four‐hour blood pressure monitoring to predict and assess impact of renal denervation: the DENERHTN Study (Renal Denervation for Hypertension). Hypertension. 2017;69:494–500. [DOI] [PubMed] [Google Scholar]
- 4. Persu A, Kjeldsen S, Staessen JA, Azizi M. Renal denervation for treatment of hypertension: a second start and new challenges. Curr Hypertens Rep. 2016;18:6. [DOI] [PubMed] [Google Scholar]
- 5. Mahfoud F, Böhm M, Azizi M, Pathak A, Durand Zaleski I, Ewen S, Tsioufis K, Andersson B, Blankestijn PJ, Burnier M, Chatellier G, Gafoor S, Grassi G, Joner M, Kjeldsen SE, Lüscher TF, Lobo MD, Lotan C, Parati G, Redon J, Ruilope L, Sudano I, Ukena C, van Leeuwen E, Volpe M, Windecker S, Witkowski A, Wijns W, Zeller T, Schmieder RE. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J. 2015;36:2219–2227. [DOI] [PubMed] [Google Scholar]
- 6. Cecelja M, Hussain T, Greil G, Botnar R, Preston R, Moayyeri A, Spector TD, Chowienczyk P. Multimodality imaging of subclinical aortic atherosclerosis: relation of aortic stiffness to calcification and plaque in female twins. Hypertension. 2013;61:609–614. [DOI] [PubMed] [Google Scholar]
- 7. McEniery CM, McDonnell BJ, So A, Aitken S, Bolton CE, Munnery M, Hickson SS, Yasmin , Maki‐Petaja KM, Cockcroft JR, Dixon AK, Wilkinson IB. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension. 2009;53:524–531. [DOI] [PubMed] [Google Scholar]
- 8. Mancia G, Giannattasio C. Diagnostic and therapeutic problems of isolated systolic hypertension. J Hypertens. 2015;33:33–43. [DOI] [PubMed] [Google Scholar]
- 9. Ewen S, Ukena C, Linz D, Kindermann I, Cremers B, Laufs U, Wagenpfeil S, Schmieder RE, Böhm M, Mafhoud F. Reduced effect of percutaneous renal denervation on blood pressure in patients with isolated systolic hypertension. Hypertension. 2015;65:193–199. [DOI] [PubMed] [Google Scholar]
- 10. Mahfoud F, Bakris G, Bhatt DL, Esler M, Ewen S, Fahy M, Kandzari D, Kario K, Mancia G, Weber M, Böhm M. Reduced blood pressure‐lowering effect of catheter‐based renal denervation in patients with isolated systolic hypertension: data from SYMPLICITY HTN‐3 and the Global SYMPLICITY Registry. Eur Heart J. 2017;38:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mory B, Ardon R, Yezzi A, Thiran J. Non‐Euclidean image‐adaptative radial basis functions for 3D interactive segmentation. 2009 IEEE 12th Int Conf Comput Vis Kyoto Jpn 2009;787–794.
- 12. Reference Values for Arterial Stiffness’ Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values”. Eur Heart J. 2010;31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stea F, Bozec E, Millasseau S, Khettab H, Boutouyrie P, Laurent S. Comparison of the Complior Analyse device with Sphygmocor and Complior SP for pulse wave velocity and central pressure assessment. J Hypertens. 2014;32:873–880. [DOI] [PubMed] [Google Scholar]
- 14. Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089–2100. [DOI] [PubMed] [Google Scholar]
- 15. Bastos Goncalves F, Voûte M, Hoeks S, Chonchol M, Boersma E, Stolker R, Verhagen JJ. Calcification of the abdominal aorta as an independent predictor of cardiovascular events. Heart. 2012;98:988–994. [DOI] [PubMed] [Google Scholar]
- 16. London GM. Mechanisms of arterial calcifications and consequences for cardiovascular function. Kidney Int Suppl. 2013;3:442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Courand PY, Milon H, Bricca G, Khettab F, Lantelme P. Diastolic blood pressure, aortic atheroma, and prognosis in hypertension: new insights into a complex association. Atherosclerosis. 2014;233:300–306. [DOI] [PubMed] [Google Scholar]
- 18. Harbaoui B, Montoy M, Charles P, Boussel L, Liebgott H, Girerd N, Courand PY, Lantelme P. Aorta calcification burden: towards an integrative predictor of cardiac outcome after transcatheter aortic valve implantation. Atherosclerosis. 2016;246:161–168. [DOI] [PubMed] [Google Scholar]
- 19. Wilson PWF, Kauppila LI, O'Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. [DOI] [PubMed] [Google Scholar]
- 20. Jensky NE, Criqui MH, Wright MC, Wassel CL, Brody SA, Allison MA. Blood pressure and vascular calcification. Hypertension. 2010;55:990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, Flack JM, Katzen BT, Lea J, Lee DP, Leon MB, Ma A, Massaro J, Mauri L, Oparil S, O'Neill WW, Patel MR, Rocha‐Singh K, Sobotka PA, Svetkey L, Townsend RR, Bakris GL. Predictors of blood pressure response in the SYMPLICITY HTN‐3 trial. Eur Heart J. 2015;36:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ott C, Schmid A, Toennes S, Ditting T, Veelken R, Uder M, Schmieder RE. Central pulse pressure predicts BP reduction after renal denervation in patients with treatment‐resistant hypertension. EuroIntervention. 2015;11:110–116. [DOI] [PubMed] [Google Scholar]
- 23. Esler M. Renal denervation for treatment of drug‐resistant hypertension. Trends Cardiovasc Med. 2015;25:107–115. [DOI] [PubMed] [Google Scholar]
- 24. Palmer BF. Renal dysfunction complicating the treatment of hypertension. N Engl J Med. 2002;347:1256–1261. [DOI] [PubMed] [Google Scholar]
- 25. O'Rourke MF, Safar ME, Dzau V. The Cardiovascular Continuum extended: aging effects on the aorta and microvasculature. Vasc Med. 2010;15:461–468. [DOI] [PubMed] [Google Scholar]
- 26. DiBona GF, Sawin LL. Effect of renal denervation on dynamic autoregulation of renal blood flow. Am J Physiol Renal Physiol. 2004;286:F1209–F1218. [DOI] [PubMed] [Google Scholar]
- 27. DiBona GF. Physiology in perspective: the wisdom of the body. Neural control of the kidney. Am J Physiol Regul Integr Comp Physiol. 2005;289:R633–R641. [DOI] [PubMed] [Google Scholar]
- 28. Linz D, Hohl M, Schütze J, Mahfoud F, Speer T, Linz B, Hübschle T, Juretschke HP, Deschend R, Geisel J, Rütten H, Böhm M. Progression of kidney injury and cardiac remodeling in obese spontaneously hypertensive rats: the role of sympathetic innervation. Am J Hypertens. 2015;28:256–265. [DOI] [PubMed] [Google Scholar]
- 29. Zhang Z, Yang K, Zeng L, Wang X, Jiang F, Tu S, Liang Q, Shen Z. Renal simplicity denervation reduces blood pressure and renal injuries in an obesity‐induced hypertension dog model. Clin Exp Pharmacol Physiol. 2016. Aug 25. DOI: 10.1111/1440-1681.12661. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30. Doltra A, Hartmann A, Stawowy P, Goubergrits L, Kuehne T, Wellnhofer E, Gebker R, Schneeweis C, Schnackenburg B, Esler M, Fleck E, Kelle S. Effects of renal denervation on renal artery function in humans: preliminary study. PLoS One. 2016;11:e0150662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, Linz D, Schmieder RE, Rump LC, Kindermann I. Renal hemodynamics and renal function after catheter‐based renal sympathetic denervation in patients with resistant hypertension. Hypertension. 2012;60:419–424. [DOI] [PubMed] [Google Scholar]
- 32. Vink E, Boer A, Verloop W, Spiering W, Voskuil M, Vonken E, Hoogduin JM, Leiner T, Bots ML, Blankestijn PJ. The effect of renal denervation on kidney oxygenation as determined by BOLD MRI in patients with hypertension. Eur Radiol. 2015;25:1984–1992. [DOI] [PubMed] [Google Scholar]
- 33. Ott C, Janka R, Schmid A, Titze S, Ditting T, Sobotka PA, Veelken R, Uder M, Schmieder RA. Vascular and renal hemodynamic changes after renal denervation. Clin J Am Soc Nephrol. 2013;8:1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ott C, Mahfoud F, Schmid A, Toennes SW, Ewen S, Ditting T, Veelken R, Ukena C, Uder M, Böhm M, Schmieder RE. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J Hypertens. 2015;33:1261–1266. [DOI] [PubMed] [Google Scholar]
- 35. Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon BM, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha‐Singh K, Townsend RR, Bakris GL. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. [DOI] [PubMed] [Google Scholar]
- 36. Id D, Kaltenbach B, Bertog SC, Hornung M, Hofmann I, Vaskelyte L, Sievert H. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC Cardiovasc Interv. 2013;6:1085–1091. [DOI] [PubMed] [Google Scholar]
- 37. McCambridge J, Witton J, Elbourne D. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Esler M, Böhm M, Sievert H, Rump C, Schmieder R, Krum H, Mahfoud F, Schlaich M. Catheter‐based renal denervation for treatment of patients with treatment‐resistant hypertension: 36 month results from the SYMPLICITY HTN‐2 randomized clinical trial. Eur Heart J. 2014;35:1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laffin L, Bakris G. Renal denervation: a filed in flux. Curr Hypertens Rep. 2016;18:56. [DOI] [PubMed] [Google Scholar]


