Figure 1.
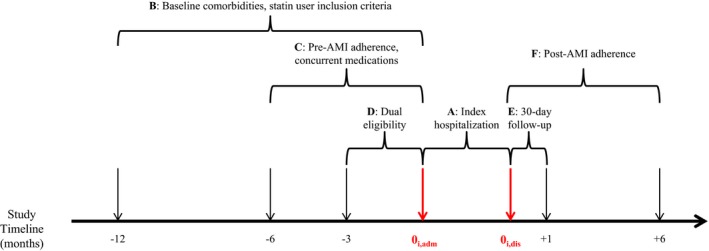
Study timeline. A, Index hospitalization (duration is length of stay). B, Twelve‐month period used to identify baseline comorbidities. This period was used to identify prevalent users of statins for study inclusion. C, Six‐month period used to identify concurrent users of angiotensin‐converting (ACE) enzyme inhibitors/angiotensin receptor blockers (ARB) and β‐blockers. Also used to measure pre–acute myocardial infarction (AMI) statin adherence. If a patient's first prescription claim occurred during this period, adherence was measured from the date of that first fill until the first day of hospital admission for index AMI (0i,adm). D, Three‐month period used to identify patients with dual Medicare and Medicaid eligibility. If a patient had dual eligibility during any of these 3 months, they were considered dual eligible for the entire study. E, Thirty‐day period after index hospitalization discharge used to measure whether patient followed up with a primary care provider and/or cardiologist. F, Follow‐up period for all patients used to measure post‐AMI statin adherence. This period lasted 6 months after hospital discharge except for those individuals who died within 6 months of hospital discharge (n=12 281, 10.8%). Date of death was the end of follow‐up for these patients. 0i,dis indicates discharge date for index hospitalization (which was the beginning of the follow‐up period for all patients).
