Abstract
Background:
Hormesis is considered a dose–response phenomenon characterized by growth stimulation at low doses and inhibition at high doses. The hormetic response by silver nanoparticles (AgNPs) on in vitro multiplication of sugarcane was evaluated using a temporary immersion system.
Methods:
Sugarcane shoots were used as explants cultured in Murashige and Skoog medium with AgNPs at concentrations of 0, 25, 50, 100, and 200 mg/L. Shoot multiplication rate and length were used to determine hormetic response. Total content of phenolic compounds of sugarcane, mineral nutrition, and reactive oxygen species (ROS) was determined.
Results:
Results were presented as a dose–response curve. Stimulation phase growth was observed at 50 mg/L AgNPs, whereas inhibition phase was detected at 200 mg/L AgNPs. Mineral nutrient analysis showed changes in macronutrient and micronutrient contents due to the effect of AgNPs. Moreover, AgNPs induced ROS production and increased total phenolic content, with a dose-dependent effect.
Conclusion:
Results suggested that the production of ROS and mineral nutrition are key mechanisms of AgNP-induced hormesis and that phenolic accumulation was obtained as a response of the plant to stress produced by high doses of AgNPs. Therefore, small doses of AgNPs in the culture medium could be an efficient strategy for commercial micropropagation.
Keywords: hormesis, in vitro regeneration, phenolic compounds, plant nutrition, reactive oxygen species
Introduction
Advances in nanotechnology that have been integrated into biology have led to the emergence of a new exciting discipline called nanobiotechnology.1 Nanoparticles (NPs: 1-100 nm in diameter) are small forms of natural or manufactured source material whose properties differ markedly from those of the respective bulk forms of the “same” material.2 Certain NPs have diagnostic and therapeutic uses; some NPs exhibit low-dose toxicity, while other NPs show ability to stimulate low-dose adaptive responses.3 The effect of dose response, characterized by stimulation at low doses and inhibition at high doses, is called hormesis.4 It has been reported that some types of NPs can initiate hormesis in plants.5–7
Silver nanoparticles (AgNPs) have been widely used in pharmaceutical, cosmetic, textile, food, and agricultural products, due to their beneficial microbicidal and antiviral properties. In plant tissue culture, AgNPs have been used to improve seed germination, enhance plant growth and yield, improve bioactive compound production, enable plant genetic modification, and achieve plant protection.8,9 Silver NPs have great influence on the growth and development of lettuce and alfalfa, among other plants; AgNPs have been shown to increase germination and growth rates, enhance root elongation, and reduce senescence in plants.10,11 Crop plants such as Phaseolus radiatus and Sorghum bicolor grown in nutrient medium supplemented with AgNPs can improve nutrient use efficiency.12 Induction of reactive oxygen species (ROS) by mild stress leading to the activation of antioxidant defenses, stress-signaling hormones, or adaptive growth responses are the most probable pathways for hormetic responses.13
Sugarcane (Saccharum spp.) is one of the most important commercial crops cultivated worldwide for the production of sugar, ethanol, and other related by-products.14 In vitro multiplication of sugarcane is important to obtain certified seedbeds for plants that are free of pathogens and genetically homogeneous. Conventional micropropagation of sugarcane in semisolid culture has been reported.15,16 However, to reduce the labor required and increase efficiency, temporary immersion systems have been successfully used to improve in vitro sugarcane multiplication.17–19 In vitro AgNPs in plants are widely used for their antimicrobial properties.20,21 However, whether AgNPs have a hormetic effect on in vitro plant multiplication has not been evaluated. The mechanism by which hormesis works in plants is not fully understood. Considering the cytotoxic reports of different AgNP formulations available, the commercial preparation Argovit (Russian and American patent 2427380 and 2014200380, respectively) was used as source material in this study. Argovit (Novosibirsk, Russia) is currently approved in Russia and other countries for use in veterinary and human applications.22 Therefore, in order to further investigate the potential applications of AgNPs, the influence of Argovit AgNPs on in vitro multiplication of sugarcane (Saccharum spp.), in terms of shoot multiplication, mineral nutrient contents, total phenolic content (TPC), antioxidant capacity, ROS production, and lipid peroxidation, was evaluated using a temporary immersion system.
Methods
Silver NP Characterization
Argovit formulation was obtained from Scientific-Production Centre Vector-Vita Ltd (Novosibirsk, Russia). Argovit consists of a solution of spherical AgNPs measuring 35 ± 15 nm in size, which is clustered silver (12 mg/mL metallic silver) functionalized with 188 mg/mL of polyvinylpyrrolidone (PVP, 10-30 kD) in water with an overall concentration of 20% AgNPs (200 mg/mL). The size of metallic AgNPs was measured using a JEOL (Tokyo, Japan) JEM-2010 high-resolution transmission electron microscope. Hydrodynamic diameter, which is composed of metallic AgNPs and PVP ligands, was measured in suspension. Argovit impurities are α-pyrrolidone ≤0.6%, N-vinylpyrrolidone ≤0.04%, aldehydes ≤0.04%, and peroxide compounds ≤0.008%. In regard to aggregation condition, excess of PVP ligands and ζ potential equal to −15 mV. Expiration of Argovit is 2 years, which means that AgNP size is maintained for 2 years. In relation to dissolution, in Argovit, there is a small concentration of silver cations, which are in equilibrium with silver metallic particles. However, the concentration of silver cations is very low due to the reduced ability of PVP ligands. In this study, a single batch was used (No. 01200-0814). Argovit physicochemical characterization is according to Juarez-Moreno et al.23 (Table 1).
Table 1.
Physicochemical Characteristics of Argovit.
| Properties | Mean |
|---|---|
| Metallic silver content (% wt.) | 1.2 |
| PVP content (% wt.) | 18.8 |
| Silver nanoparticle morphology | Spheroid |
| Average diameter of metallic silver particles by TEM data (nm) | 35 |
| Size interval of metallic silver particles by TEM data (nm) | 1 to 90 |
| Hydrodynamic diameter: metallic Ag with PVP (nm) | 70 |
| Zeta potential (mV) | −15 |
| Surface plasmon resonance | 420 nm |
| PVP structure by FTIR | Confirmed |
Abbreviations: Ag, silver; FTIR, Fourier transform infrared; PVP, polyvinylpyrrolidone; TEM, transmission electron microscopic.
Effect of AgNPs on In Vitro Shoot Multiplication
Sugarcane meristems (cv. Mex 69-290) were collected from field-grown plants and cultured following the protocol of Jiménez et al.24 Three-centimeter-long sugarcane shoots after 3 subcultures (30 days each) were used as explant. Ten explants (2 shoots each) were placed in 1-L temporary immersion bioreactors (TIBs) containing 500 mL Murashige and Skoog25 medium supplemented with 30 g/L sucrose, 1 mg/L kinetin (Sigma-Aldrich® Chemical Company, St. Louis, MO), 0.6 mg/L 3-indoleacetic acid (Sigma-Aldrich® Chemical Company, St. Louis, MO), and 0.3 mg/L 6-benzylaminopurine (Sigma-Aldrich® Chemical Company, St. Louis, MO). The pH of the culture medium was adjusted to 5.8 with 0.1 N sodium hydroxide and then autoclaved at 1.2 kg/cm2 for 15 minutes at 120°C. After sterilization, different solutions of AgNPs with various concentrations (0, 25, 50, 100, and 200 mg/L) were added to the medium. For each treatment, 3 TIBs were used. The experiment was repeated 3 times. Temporary immersion bioreactor was incubated at 24°C ± 2°C and was maintained under fluorescent light (40-50/µmoL·m2/s) and a photoperiod of 16 hours. Immersion frequency was measured according to Lorenzo et al.17 After 30 days of incubation, the number and length of shoots per explant were assessed.
Effect of AgNPs on Sugarcane Macronutrient and Micronutrient Contents
A macronutrient and micronutrient analysis was performed for each treatment after 30 days of culture. Minerals were analyzed according to Elmer26 by dry ashing 1 g of shoot leaves (sample) at 550°C. The ash obtained was dissolved in 1.5 N HCl, filtered through an acid-washed paper filter, and brought to standard volume (100 mL) with deionized water. Calcium (Ca), magnesium (Mg), potassium (K), iron (Fe), copper (Cu), zinc (Zn), and manganese (Mn) nutrients were determined using atomic absorption spectrophotometry (Perkin Elmer Analyst 400, Waltham, MA). Phosphorus (P) content was determined by employing the vanado-molybdate method and measured with Perkin Elmer Lambda 25 UV−Vis colorimeter at 630 nm.27 Boron (B) content was determined by the spectrophotometric method with curcumin at 540 nm. Total nitrogen was determined by digestion with sulfuric acid followed by distillation with NaOH according to the micro-Kjeldahl method.28 For all nutrients, the analysis was performed in triplicate.
Effect of AgNPs on Sugarcane TPC, Antioxidant Capacity, ROS Production, and Lipid Peroxidation
For TPC, 2 g of shoot leaves were extracted for 3 hours at 250 rpm with 50% methanol in water (vol/vol) using a mass–solvent ratio of 1:10 (wt/vol) at 30°C. The supernatant was recovered and filtered with a vacuum pump through a Whatman 1 filter paper. The extract was concentrated in a rotary evaporator to remove methanol. After the methanol had been removed, the extract was lyophilized, and the resulting freeze-dried powder was stored at −80°C. The TPC of the sugarcane shoots was examined using the Folin–Ciocalteu method described by Payet et al.29 Briefly, appropriate dilutions of freeze-dried extract in methanol were transferred to a 96-well microplate (Nunc, Roskilde, Denmark) and oxidized with Folin–Ciocalteu reagent at room temperature for 5 minutes. After that, sodium carbonate (2%) was added to each well. The microplate was immediately placed and agitated in a microplate reader (Synergy HT; Bio-Tek, Winooski, Vermont) and then allowed to stand for 15 minutes. The absorbance was measured at 760 nm, and TPC was calculated from a calibration curve of gallic acid and expressed as milligrams of gallic acid equivalents per gram sample.
Antioxidant capacity oxygen radical absorbance capacity (ORAC) was determined according to the method described by Huang et al.30 One gram of freeze-dried extract of sugarcane shoots was diluted in methanol for quantification. The peroxide radicals were produced by 2,2′-azobis(2-amidinopropane) dihydrochloride, using fluorescein as substrate and Trolox as standard. Fluorescence was measured every 2 minutes for 1 hour, and a calibration curve of Trolox at different concentrations (from 10 to 100 μM) was used in each plate read.
The determination of ROS was performed by a direct colorimetric and fluorometric assay that measures hydrogen peroxide (H2O2) as a reactive oxygen metabolic by-product. Hydrogen peroxide assay kit (ab102500; CRT Scientific, Mexico City, Mexico) was used. The supernatant was collected, transferred to a clean tube, and kept on ice for deproteination with perchloric acid (PCA). The deproteinized samples were used for the H2O2 assay kit. To calculate the original concentration, a dilution factor of final sample was calculated, taking into account initial sample volume + vol PCA + vol Potassium hydroxide (KOH).
To evaluate lipid peroxidation, 200 mg of freeze-dried sugarcane shoots were homogenized in 4 mL of 0.1% trichloroacetic acid (TCA). Then, the extract was centrifuged at 10 000g for 15 minutes, and the supernatant (1 mL) was collected and mixed with 2 mL of 20% TCA and 2 mL of 0.5% thiobarbituric acid. The absorbance of supernatant was read at 532 and 600 nm (Synergy HT; Bio-Tek). The concentration of the malondialdehyde (MDA), formed by the decomposition of polyunsaturated fatty acids, was calculated using Beer-Lambert’s equation. All assays were carried out in triplicate.
Statistical Analysis
The experimental design was completely randomized. To compare the number and length of shoots based on the AgNP concentration (treatments), we used 2 types of statistical models. A generalized linear mixed model for Poisson response was used for shoot number and a linear mixed model for shoot length.
Shoot Number
Linear predictor
The linear predictor of these data is , where ηij is the ith link for the treatment i and the replication j,η, is the intercept, τi is the ith fixed treatment effect, and rj is the jth random effect due to repetitions.
Distribution
The response has a Poisson distribution, that is, , and each .
Link function
and the inverse for each link is .31
Shoot Length
Linear predictor
The linear predictor of these data is , where yij is the shoot length for the treatment i and the repetition j, μ is the overall mean, τi is the ith fixed treatment effect, and rj is the jth random effect due to repetitions.
Distribution
The response has a normal distribution, that is, , and .
Hormetic Effect
To test whether the responses (number and length of shoots) are hormetic, we fitted our data using the Brain and Cousens model32:
where yij denotes the response for i NP concentration at the j repetition, xij is the ijth concentration level of NPs, δ denotes the rate response at infinite doses, α denotes the mean response of the untreated control, θ, γ denotes the degree of hormetic increase , and the size of β does so thereafter.
The statistical analysis was performed by using 2 SAS procedures (Statistical Analysis System version 9.2), the PROC GLIMMIX and PROC NLIN and SPSS v. 22 for Windows, and means were compared with the Tukey test (P ≤.05). Arcsine transformation was performed for the percentages of macronutrients and micronutrients before subjecting them to statistical analysis.
Results
Effect of AgNPs on Sugarcane In Vitro Shoot Multiplication
In vitro shoot multiplication and elongation showed significant differences for treatments with different AgNP concentrations (Table 2). The greatest shoot number and length were obtained in the treatment with 50 and 100 mg/L AgNPs. The lowest AgNP concentration (25 mg/L) had no effect on the evaluated variables. However, the highest AgNP concentration caused a reduction in shoot number and length (Figure 1).
Table 2.
Effect of Silver Nanoparticle Concentration on In Vitro Shoot Multiplication of Sugarcane cv. Mex 69-290 After 30 Days of Culture in Temporary Immersion Bioreactors.a
| AgNPs (mg/L) | No. of Shoots | Shoot Length (cm) |
|---|---|---|
| 0 | 34.90 ± 1.52b,c | 3.65 ± 0.25b |
| 25 | 38.90 ± 1.61c | 4.31 ± 0.25b |
| 50 | 47.28 ± 1.69d | 5.55 ± 0.24d |
| 100 | 44.90 ± 1.69d | 5.41 ± 0.25d |
| 200 | 31.97 ± 1.30c | 2.62 ± 0.23c |
aMeans ± standard error within a column followed by the same letter (b, c, or d) are not significantly different according to Tukey test at P ≤.05.
Figure 1.
Effect of silver nanoparticle (AgNPs) concentration on in vitro shoot multiplication of sugarcane cv. Mex 69-290 after 30 days of culture in temporary immersion bioreactors. A-E, 0, 25, 50, 100, and 200 mg/L of AgNPs, respectively. Bar = 1 cm.
Effect of AgNPs on Sugarcane Macronutrient and Micronutrient Contents
Effect of AgNP concentration on macronutrient and micronutrient contents is presented in Table 3. The concentrations of the macronutrients N and Mg in the shoots were increased compared to the control plants. Phosphorus, K, and Ca did not show any difference among the treatments. On the other hand, Fe and B were the only micronutrients with differences caused by the treatment with AgNPs. The shoot concentrations of Cu, Zn, and Mn did not show any significant differences among the treatments. The treatment with 50, 100, and 200 mg/L AgNPs showed a significant increase in N, Mg, and Fe content. The only depletion in nutrient use efficiency was detected for B in AgNP treatments.
Table 3.
Effect of Silver Nanoparticles on Macronutrient and Micronutrient Contents in Shoots of Sugarcane cv. Mex 69 to 290 After 30 Days of Culture in Temporary Immersion Bioreactors.a
| AgNPs (mg/L) | Macronutrients (% of wt) | Micronutrients (mg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | Fe | Cu | Zn | Mn | B | |
| 0 | 2.57 ± 0.59b | 0.059± 0.00b | 0.65 ± 0.19b | 0.28 ± 0.02b | 0.12 ± 0.00d | 17.09 ± 0.21c | 1.42 ± 0.11b | 55.93 ± 21.16b | 89.08 ± 36.51b | 37.35 ± 0.91b |
| 25 | 3.83 ± 0.23b | 0.054± 0.00c | 0.40 ± 0.04c | 0.35 ± 0.09c | 0.15 ± 0.00b | 18.02 ± 0.28b | 1.63 ± 0.18c | 67.40 ± 32.32c | 102.79 ± 43.78c | 12.03 ± 0.01b |
| 50 | 5.62 ± 0.14b | 0.045± 0.00c | 0.31 ± 0.01c | 0.38 ± 0.11a | 0.17 ± 0.00b,c | 23.80 ± 0.16c | 1.41 ± 0.08c | 66.95 ± 33.17c | 101.09 ± 48.24c | 14.82 ± 1.97b |
| 100 | 5.73 ± 0.13c | 0.047± 0.00c | 0.46 ± 0.09c | 0.41 ± 0.13c | 0.17 ± 0.00b,c | 25.38 ± 0.99c | 1.71 ± 0.08c | 63.01 ± 28.57c | 97.73 ± 34.49c | 14.43 ± 1.24b |
| 200 | 6.11 ± 0.49c | 0.048± 0.00c | 0.51 ± 0.11c | 0.34 ± 0.08c | 0.18 ± 0.00c | 24.84 ± 2.00c | 1.32 ± 0.10c | 59.85 ± 23.90c | 103.18 ± 35.80c | 15.25 ± 1.48b |
Abbreviations: B, boron; Ca, calcium; Cu, copper; Fe, Iron; K, potassium; Mg, magnesium; Mn, Manganese; N, total nitrogen; P, phosphorus; Zn, Zinc.
aMeans ± standard error within a column followed by the same letter (b, c, or d) are not significantly different according to Tukey test at P ≤.05.. ;.
Effect of AgNPs on Sugarcane TPC, Antioxidant Capacity, ROS Production, and Lipid Peroxidation
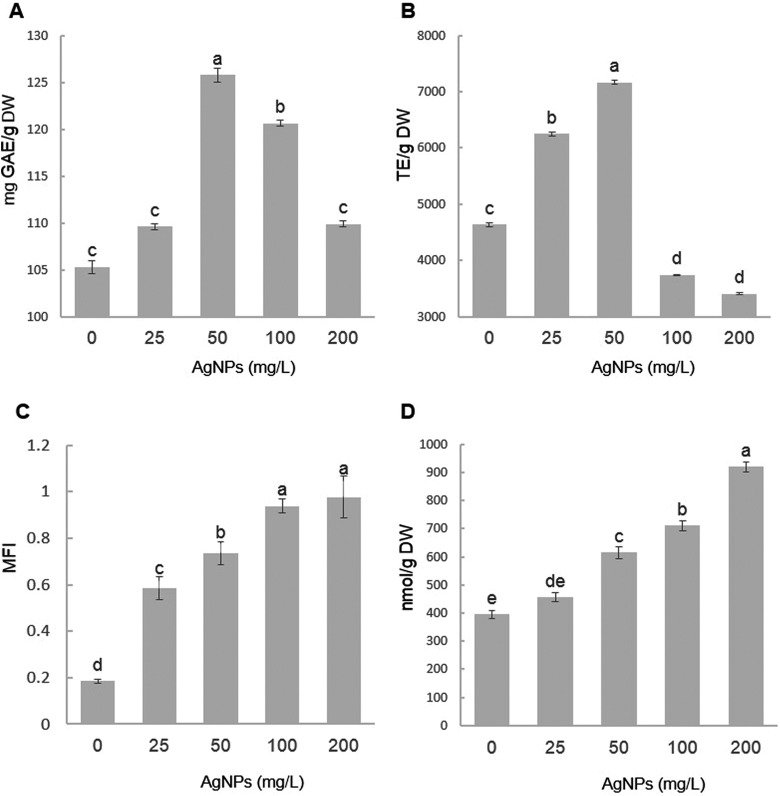
Figure 2 shows the results of the effect of different AgNP concentrations on TPC, ORAC, ROS, and lipid peroxidation-MDA (LP-MDA) during sugarcane micropropagation. Differences were found in TPC, ORAC, ROS, and LP-MDA contents of sugarcane shoots obtained in different treatments with AgNPs. Sugarcane shoots treated with 50 mg/L AgNPs had a significant increase in TPC content. The highest ORAC was obtained in the 25 and 50 mg/L AgNP treatments, whereas the lowest was obtained with 100 and 200 mg/L AgNPs. Results show a relationship between shoot number and length with antioxidant activity induced by AgNPs. For ROS and LP-MDA generation, results show that both indicators increased significantly as the AgNP concentration in the culture medium was increased. The highest values of these indicators were observed at 100 and 200 mg/L AgNPs.
Figure 2.
Effect of silver nanoparticle concentration on (A) total phenolic content (TPC), (B) antioxidant capacity (ORAC), (C) reactive oxygen species (ROS) production, and (D) lipid peroxidation (LP-MDA) of sugarcane cv. Mex 69-290 after 30 days of in vitro multiplication of shoots in temporary immersion bioreactors (TPC). Temporary immersion bioreactors expressed as milligrams of gallic acid equivalents per gram of dry weight (mg GAE/gDW); ORAC quantified by oxygen radical absorbance capacity expressed as Trolox equivalents per gram of dry weight (TE/g DW); ROS expressed as mean fluorescence intensity (MFI); LP-MDA quantified by malondialdehyde assay (MDA), expressed as nanomole per gram of dry weight (nmoL/gDW). Different letters denote statistically significant differences according to Tukey test (P ≤.05).
Hormetic Response
The hormetic effect on shoot number and length is represented in the hormetic growth response curve in Figure 3. According to the hormetic response curve, the maximum stimulatory response for shoot number and length occurs at 50 and 100 mg/L AgNPs (parameter A). The hormetic zone was observed at concentrations between 16.81 and 123.12 mg/L and between 11.99 and 104.77 mg/L for shoot number and length, respectively (parameter B). This parameter represents the stimulatory region that is ≥110% of the control. Control responses were 34.90 ± 1.52 and 3.65 ± 0.25 for shoot number and length, respectively (parameter C). Finally, toxic threshold was observed at concentrations of 200 mg/L AgNPs (parameter D). This parameter is defined as the concentration where the response is equal to the control.
Figure 3.
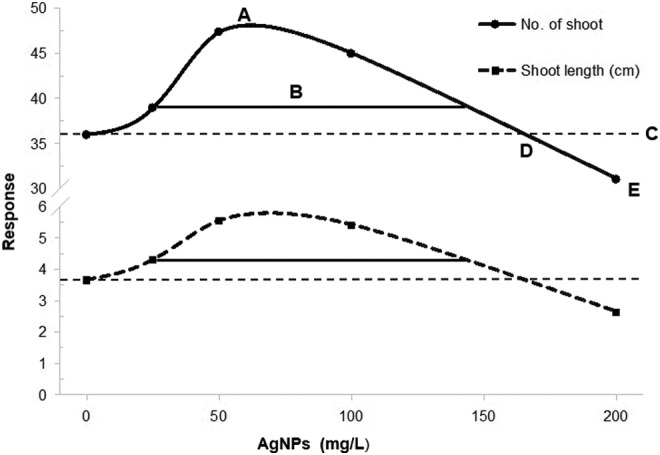
Hormetic curve response by silver nanoparticle concentration on in vitro shoot multiplication and shoot length of sugarcane cv. Mex 69-290 after 30 days of in vitro culture on temporary immersion bioreactors. (A) Maximum stimulatory response; (B) hormetic zone, the width of the stimulatory response calculated from the lowest (P 1) to the highest (P 2) concentration showing a response that is 110% of the control response (C) and (D) toxic threshold.
Discussion
Effect of AgNPs on Sugarcane In Vitro Shoot Multiplication
Silver NP concentrations evaluated in this study showed significant differences in shoot multiplication and elongation. The 50 and 100 mg/L concentrations of AgNPs increased shoot number and length, whereas the highest concentration (200 mg/L) inhibited both processes. The increase in the multiplication rate may be due to the increase in important nutrients such as N, Mg, and Fe. These elements are associated with the biosynthesis of chlorophyll, an essential molecule in photosynthesis during plant growth. On the other hand, Yin et al33 suggest that AgNP-induced damage may cause the loss of gravitropism in roots through disruption of auxin transport. One of the effects of auxins is related to apical dominance, and by breaking it, the formation of new shoots is favored. However, using AgNPs at high concentrations not only affected auxin transport but also caused phytotoxicity. Toxicity caused by the silver ion probably reduced the number and length of shoots.
The fact that low AgNP concentrations have a favorable effect on plant development has been previously observed. In Vigna radiata, AgNPs at 50 mg/L were observed to dramatically increase total chlorophyll, chl-a, chl-b, and root fresh weight.34 It has also been reported that AgNPs in Glycine max favor seed germination and seedling growth.35
On the other hand, it has been reported that AgNPs at high concentrations have an adverse effect on development. Stampoulis et al36 found that in Cucurbita pepo, AgNPs at 100 and 500 mg/L reduced plant biomass and transpiration by 41% and 57%, respectively. Similarly, Zuverza-Mena et al37 reported that 500 mg/L AgNPs reduced shoot and root length by 47.7% and 40% respectively, compared to the control in Raphanus sativus plants. Similar results were reported by Amooaghaie et al38 who found that 100 mg/L AgNPs reduced germination in Brassica nigra. In Solanum tuberosum, Homaee and Ehsanpour39 found that 2 mg/L AgNPs improved dry weight, root length, and leaf area.
Effect of AgNPs on Sugarcane Macronutrient and Micronutrient Contents
Sugarcane shoots grown in culture media with AgNPs accumulated a larger amount of N, Mg, and Fe, compared to the control treatment. Probably, these elements contribute to the increase in shoot number and length. Nitrogen is a constituent of important molecules such as chlorophyll, proteins, nucleic acids, and hormones.40 Magnesium is found in the porphyrin moiety of the chlorophyll molecule and is critical to reactions involving adenosine triphosphate, whereas Fe is important in the catalytic group for redox enzymes and is required for chlorophyll biosynthesis.
Boron was the only micronutrient with a reduction in shoots caused by the treatment with AgNPs. Boron reduction could be due to the immobilization of this element in the medium by the adsorption capacities of NPs.41 There is insufficient information to understand how AgNPs affect nutrient absorption. Zuverza-Mena et al37 suggest that it is possible that AgNPs physically block the diffusion pathway or the channels for active absorption.
Shoots obtained in this study showed no significant differences in P, K, Ca, Cu, Zn, and Mn contents. This is probably due to the immobilization of these elements in culture media or because they are not required during stress metabolism. In hydroponic systems, Martínez-Fernández et al41 reported that in Helianthus annuus, the concentration of Ca, K, Mg, and S decreases in plants treated with 100 mg/L of iron oxide NPs. On the other hand, Jhanzab et al12 found that applying 25 mg/L AgNPs significantly enhanced growth and yield attributes and N-P-K uptake in Triticum aestivum.
Effect of AgNPs on Sugarcane TPC, Antioxidant Capacity, ROS Production, and Lipid Peroxidation
The results coincide with the phenolic compound and antioxidant capacity ranges previously reported for sugarcane.42,43 These results suggest that applying 50 mg/L AgNPs in the culture medium stimulates TPC production and accumulation. Iavicoli et al44 mention that at the cell level NPs can enhance the formation of ROS, disrupt the electron/ion cell membrane transport activity, and induce oxidative damage and lipid peroxidation. According to Carlson et al,45 AgNPs can cause ROS, in response to which plants raise the production of phenolic compounds with high antioxidant capacity. In Solanum tuberosum, Homaee and Ehsanpour39 observed a dose-dependent effect since increasing the AgNP concentration increased the anthocyanin content. The results obtained in this study suggest that sugarcane plants increase their production of phenolic compounds to counteract the effect of ROS. However, in the 100 and 200 mg/L concentrations, the production of phenolic compounds and antioxidant capacity decrease, while ROS production continues to rise. Lopéz-Moreno et al46 mention that the increase in ROS within the cellular membrane is caused by external factors that induce stress to the plant. The increase in ROS within the membranes due to heavy metals, such as silver, can cause considerable damage, disrupting normal cellular activity. In this sense, the results suggest that high AgNP concentrations in sugarcane induce oxidative stresses and this is probably why the number and length of shoots decrease (Figure 1). On the other hand, the destabilization of the cell membrane by lipid peroxidation is an important mechanism by which ROS can cause cell death.46 In this study, lipid peroxidation increased as AgNP concentrations increased.
Hormesis
This study demonstrated the effect of AgNPs on sugarcane shoot production and length. This effect is represented in the horticultural growth response curve. Shoot production increases by 35% and 28% at 50 and 100 mg/L AgNPs, respectively, whereas shoot length increases by 52% and 48% at 50 and 100 mg/L AgNPs, respectively. Calabrese and Blain47 and Iavicoli et al44 mention that hormetic responses display a moderate stimulatory response in the low-dose zone, with the maximum response being about 30% to 60%, which is greater than the control. The observed hormetic response can be explained by the following mechanism: At the beginning, the increase in shoot production and length from 25 mg/L AgNPs is probably due to the greater accumulation of N, Mg, and Fe. This same behavior was found at the 50 and 100 mg/L AgNPs where high antioxidant capacity counteracted the oxidative stress generated by ROS. Finally, at 200 mg/L AgNPs, the ROS-induced oxidative stress continued to increase while the antioxidant capacity decreased leading to the reduction in shoot number and length.
According to Poschenrieder et al,13 it is recommended that the term hormesis be used in plant toxicology as a descriptive term for the stimulated phase in growth response curves that is induced by low concentrations of toxic metal ions without evidence of the underlying mechanisms. Hormesis is increasingly understood as a dynamic adaptive response or biological plasticity of a complex living system at the level of the whole organism to intermittent mild stressors of various categories.48 Calabrese and Mattson49 have used hormesis as a quantitative estimate of biological plasticity due to adaption through the activation of defenses by cross signaling. The hormetic effect of AgNPs formulated as Argovit has also been shown during in vitro regeneration of Vanilla planifolia by Spinoso-Castillo et al,7 further demonstrating its use as a microbicide agent.
Conclusion
The results demonstrated that AgNPs in culture medium significantly affect in vitro parameters of development, nutrient contents, antioxidant capacity, and ROS generation of sugarcane. It was revealed that the N, Mg, and Fe contents in the shoots increased significantly (by 138%, 50%, and 47%, respectively) in the treatments with AgNPs compared to the control treatment. Oxidative stress caused by the AgNPs increased ROS production and lipid peroxidation. As a response mechanism, antioxidant capacity also increased, up to the 50 mg/L concentration. From this concentration, the antioxidant capacity decreased and this probably caused the decrease in shoot number and length. In this study, the application of 50 mg/L AgNPs was the most appropriate concentration to increase shoot production and length. Results obtained in this study open up the possibility of applying Argovit to evaluate the potential of using it in plant tissue culture. Further research is required on the effect of AgNPs on toxicological and somaclonal variation during in vitro culture and on the role of AgNPs on ethylene synthesis and transport mechanisms in plant tissues.
Footnotes
Authors’ Note: All authors listed have made substantial, direct, and intellectual contribution to the work and approved it for publication.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the International Bionanotechnology Network–CONACyT (Consejo Nacional de Ciencia y Tecnología) of Mexico. Tomsk Polytechnic University Competitiveness Enhancement Program, grant VIU-TOVPM-316/2017.
References
- 1. Razzaq A, Ammara R, Jhanzab HM, Mahmood T, Hafeez A, Hussain S. A novel nanomaterial to enhance growth and yield of wheat. J Nanosci Technol. 2015;2(1):55–58. [Google Scholar]
- 2. Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):MR17–MR71. [DOI] [PubMed] [Google Scholar]
- 3. Bell IR, Ives JA, Wayne BJ. Nonlinear effects of nanoparticles: biological variability from hormetic doses, small particle sizes, and dynamic adaptive interactions. Dose Response. 2014;12(2):202–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calabrese EJ. The maturing of hormesis as a credible dose-response model. Nonlinearity Biol Toxicol Med. 2003;1(3):319–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iavicoli I, Calabrese EJ, Nascarella MA. Exposure to nanoparticles and hormesis. Dose Response. 2010;8(4):501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calabrese EJ. Preconditioning is hormesis part II: how the conditioning dose mediates protection: dose optimization within temporal and mechanistic frameworks. Pharmacol Res. 2016;110:265–275. [DOI] [PubMed] [Google Scholar]
- 7. Spinoso-Castillo JL, Chavez-Santoscoy RA, Bogdanchikova N, Pérez-Sato JA, Morales-Ramos V, Bello-Bello JJ. Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tiss Organ Cult. 2017;129(2):195–207. [Google Scholar]
- 8. Kim DH, Gopal J, Sivanesan L. Nanomaterials in plant tissue culture: the disclosed and undisclosed. RSC Adv. 2017;7(58):36492–36505. [Google Scholar]
- 9. Ruttkay-Nedecky B, Krystofova O, Nejdl L, Adam VJ. Nanoparticles based on essential metals and their phytotoxicity. Nanobiotechnol. 2017:15(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah V, Belozerova I. Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut. 2009;197(1-4):143–148. [Google Scholar]
- 11. Ma Y, Kuang L, He X, et al. Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere. 2010;78(3):273–279. [DOI] [PubMed] [Google Scholar]
- 12. Jhanzab HM, Razzaq A, Jilani G, Rehman A, Hafeez A, Yasmeen F. Silver nano-particles enhance the growth, yield and nutrient use efficiency of wheat. Int J Agron Agri Res. 2015;7(1):15–22. [Google Scholar]
- 13. Poschenrieder C, Cabot C, Martos S, Gallego B, Barceló J. Do toxic ions induce hormesis in plants? Plant Sci. 2013;212:15–25. [DOI] [PubMed] [Google Scholar]
- 14. Barnabas L, Ramadass A, Amalraj RS, Palaniyandi M, Rasappa V. Sugarcane proteomics: an update on current status, challenges, and future prospects. Proteomics. 2015;15(10):1658–1670. [DOI] [PubMed] [Google Scholar]
- 15. Sahoo DP, Samantrai D, Rout GR. Rapid clonal propagation of Saccharum officinarum L. Vars. CO-6907 and CO-86249 and to assess the genetic uniformity through molecular markers. Plant Biosyst. 2011;145(2):445–451. [Google Scholar]
- 16. Kaur A, Sandhu JS. High throughput in vitro micropropagation of sugarcane (Saccharum officinarum L.) from spindle leaf roll segments: cost analysis for agri-business industry. Plant Cell Tiss Organ Cult. 2015;120(1):339–350. [Google Scholar]
- 17. Lorenzo JC, González BL, Escalona M, Teisson C, Borroto C. Sugarcane shoot formation in an improved temporary immersion system. Plant Cell Tiss Organ Cult. 1998;54(3):197–200. [Google Scholar]
- 18. Rodriguez R, Cid M, Pina D, Gonzalez-Olmedo JL, Desjardins Y. Growth and photosynthetic activity during acclimatization of sugarcane plantlets cultivated in temporary immersion bioreactors. In Vitro Cell Dev Biol-Plant. 2003;39(6):657–662. [Google Scholar]
- 19. Mordocco AM, Brumbley JA, Lakshmanan P. Development of a temporary immersion system (RITATM) for mass production of sugarcane (Saccharum spp. interspecific hybrids). In Vitro Cell Dev Biol Plant. 2009;45(4):450–457. [Google Scholar]
- 20. Mahna N, Vahed SZ, Khani S. Plant in vitro culture goes nano: nanosilver-mediated decontamination of ex vitro explants. J Nanomed Nanotechnol. 2013;4(2):161 doi:10.4172/2157-7439.1000161. [Google Scholar]
- 21. Arab MM, Yadollahi A, Hosseini-Mazinani M, Bagheri S. Effects of antimicrobial activity of silver nanoparticles on in vitro establishment of G× N15 (hybrid of almond× peach) rootstock. J Genet Eng Biotechnol. 2014;12(2):103–110. [Google Scholar]
- 22. Borrego B, Lorenzo G, Mota-Morales JD, et al. Potential application of silver nanoparticles to control the infectivity of Rift Valley fever virus in vitro and in vivo. Nanomedicine. 2016;12(5):1185–1192. [DOI] [PubMed] [Google Scholar]
- 23. Juarez-Moreno KO, Gonzalez EB, Giron-Vazquez N, et al. Comparison of cytotoxicity and genotoxicity effects of silver nanoparticles on human cervix and breast cancer cell lines. Hum Exp Toxicol. 2016;36(9):1–18. doi:10.1177/0960327116675206. [DOI] [PubMed] [Google Scholar]
- 24. Jiménez E, Pérez J, Gil V, Herrera J, García Y, Alonso E. Sistema para la propagación de la caña de azúcar In: Estrade M, Riego E, Limonta E, Tellez P, Fuente J, eds. Avances en Biotecnología Moderna. Vol 3 La Habana, Cuba: Elfos Scientiae; 1995. [Google Scholar]
- 25. Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473–497. [Google Scholar]
- 26. Elmer P. Atomic Absorption Spectroscopy Analytical Methods. Waltham, MA: The Perkin Elmer Corporation; 1996. [Google Scholar]
- 27. Association of Official Analytical Chemists. Official Methods of Analysis of the AOAC. 16th ed Washington, DC: Association of Official Analytical Chemists; 1990. [Google Scholar]
- 28. Kjeldahl J. A new method for the determination of nitrogen in organic matter. Z Anal Chem. 1883;22(1):366–382. [Google Scholar]
- 29. Payet B, Shum A, Smadja J. Comparison of the concentrations of phenolic constituents in cane sugar manufacturing products with their antioxidant activities. J Agric Food Chem. 2006;54(19):7270–7276. [DOI] [PubMed] [Google Scholar]
- 30. Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Prior RL. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J Agric Food Chem. 2002;50(16):4437–4444. [DOI] [PubMed] [Google Scholar]
- 31. Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS® for Mixed Models. 2nd ed Cary, NC: SAS Institute Inc; 2006. [Google Scholar]
- 32. Brain P, Cousens R. An equation to describe dose responses where there is stimulation of growth at low doses. Weed Res. 1989;29(2):93–96. [Google Scholar]
- 33. Yin L, Colman BP, McGill BM, Wright JP, Bernhardt ES. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS One. 2012;7(10):e47674 doi:10.1371/journal.pone.0047674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Najafi S, Jamei R. Effect of silver nanoparticles and Pb (NO3)2 on the yield and chemical composition of mung bean (Vigna radiata). J Stress Physiol Biochem. 2014;10(1):317–325. [Google Scholar]
- 35. Lu C, Zhang C, Wen J, Wu G, Tao M. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2001;21(3):168–171. [Google Scholar]
- 36. Stampoulis D, Sinha SK, White JC. Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol. 2009;43(24):9473–9479. [DOI] [PubMed] [Google Scholar]
- 37. Zuverza-Mena N, Armendariz R, Peralta-Videa JR, Gardea-Torresdey JL. Effects of silver nanoparticles on radish sprouts: root growth reduction and modifications in the nutritional value. Front Plant Sci. 2016;7:90 doi:10.3389/fpls.2016.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Amooaghaie R, Tabatabaei F, Ahadi AM. Role of hematin and sodium nitroprusside in regulating Brassica nigra seed germination under nanosilver and silver nitrate stresses. Ecotoxicol Environ Saf. 2015;113:259–270. [DOI] [PubMed] [Google Scholar]
- 39. Homaee MB, Ehsanpour AA. Physiological and biochemical responses of potato (Solanum tuberosum) to silver nanoparticles and silver nitrate treatments under in vitro conditions. Indian J Plant Physiol. 2015;20(4):353–359. [Google Scholar]
- 40. Hopkins WG, Huner NPA. Introduction to Plant Physiology. London, Ontario: John Wiley and Sons, Inc; 2004. [Google Scholar]
- 41. Martínez-Fernández D, Barroso D, Komárek M. Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environ Sci Pollut Res. 2016;23(2):1732–1741. [DOI] [PubMed] [Google Scholar]
- 42. Lorenzo JC, De los Angeles-Blanco M, Peláez O, et al. Sugarcane micropropagation and phenolic excretion. Plant Cell Tiss Organ Cult. 2001;65(1):1–8. [Google Scholar]
- 43. Gomathi R, Rakkiyapan P. Comparative lipid peroxidation, leaf membrane thermostability, and antioxidant system in four sugarcane genotypes differing in salt tolerance. Int J Plant Physiol Biochem. 2011;3(4):67–74. [Google Scholar]
- 44. Iavicoli I, Fontana L, Leso V, Calabrese EJ. Hormetic dose-responses in nanotechnology studies. Sci Total Environ. 2014:487:361–74. [DOI] [PubMed] [Google Scholar]
- 45. Carlson C, Hussain SM, Schrand AM, et al. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112(43):13608–13619. [DOI] [PubMed] [Google Scholar]
- 46. López-Moreno ML, Avilés LL, Pérez NG, Irizarry, et al. Effect of cobalt ferrite (CoFe2O4) nanoparticles on the growth and development of Lycopersicon lycopersicum (tomato plants). Sci Total Environ. 2016;550:45–52. [DOI] [PubMed] [Google Scholar]
- 47. Calabrese EJ, Blain RB. The hormesis database: the occurrence of hormetic dose responses in the toxicological literature. Regul Toxicol Pharmacol. 2011;61(1):73–81. [DOI] [PubMed] [Google Scholar]
- 48. Calabrese EJ. Biphasic dose responses in biology, toxicology and medicine: accounting for their generalizability and quantitative features. Environ Pollut. 2013;182:452–460. [DOI] [PubMed] [Google Scholar]
- 49. Calabrese EJ, Mattson MP. Hormesis provides a generalized quantitative estimate of biological plasticity. J Cell Commun Signal. 2011;5(1):25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]