Abstract
Purpose
CDH3-related hypotrichosis with juvenile macular dystrophy (HJMD) is an autosomal-recessive entity characterized by congenital sparse scalp hair and macular dystrophy, leading to severe central visual loss. We report a family with HJMD caused by a novel CDH3 gene mutation and review the mutation spectrum in HJMD. A detailed phenotypic assessment for patients whose molecular results were reported previously is also summarized.
Observations
We present a 13-year-old Turkish girl who experienced gradual bilateral visual deterioration with marked hair loss. Hair-pull test results and scalp skin texture were normal. The eyebrows and eyelashes were normal, and no abnormality in the teeth, nails, or limbs was detected. Fundus examination revealed bilateral ring-shaped atrophy of the retinal pigment epithelium with patchy intraretinal pigment clumping at the posterior pole. DNA sequencing analysis detected a novel homozygous deletion (c.447_467del (p.149_156del)) in exon 5 of the CDH3 gene of the patient. Both healthy parents and an older brother were heterozygous for the mutation.
Conclusions and importance
This case of HJMD was related to a novel homozygous mutation, termed c.447_467del (p.149_156del). These findings have significance for the future mutational analysis and genetic counseling of families with HJMD, particularly in our region. The presence of sparse hair in childhood, with or without limb anomalies, should alert clinicians to request an eye consultation. Pediatricians, dermatologists, and ophthalmologists should be aware of the rarely seen entity of juvenile macular dystrophy with hypotrichosis.
1. Introduction
Hypotrichosis is a type of hair loss and commonly begins in early childhood.1 In hypotrichosis, sparse hair is the result of impaired regeneration, caused by defects in hair cycling and anchoring of the hair shaft in the skin.1, 2 Mutations in different genes have been associated with non-syndromic forms of hypotrichosis.3 However, hypotrichosis can also be a component of a syndrome. Hypotrichosis with juvenile macular dystrophy (HJMD; MIM601553) is among the typical forms of these disorders.2, 4 This autosomal-recessive disorder is caused by mutations in the CDH3 gene, which encodes P-cadherin and is responsible for calcium-dependent cell-to-cell adhesion.4 The gene is expressed in retinal pigment epithelium (RPE) and the hair matrix at various stages of development of these tissues.2, 4
Hypotrichosis is very rare and has been described mostly in Arab Muslim, Israeli, Turkish, and Pakistani families who reside in middle and near east countries.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Its prevalence is unknown and has only been reported in approximately 30 patients since the first case was described in 1935 by Wagner.1 It is characterized by sparse hair and progressive degeneration of the central retina, leading to legal blindness during the first 30 years of life.4
Here, we present the clinical characteristics of a case with HJMD associated with a novel CDH3 gene mutation in a non-consanguineous Turkish family. We also summarize the results of molecular analyses of 20 previously described HJMD families and discuss the detailed phenotypic presentation and genotype-phenotype correlation of patients tested molecularly.
1.1. Case report
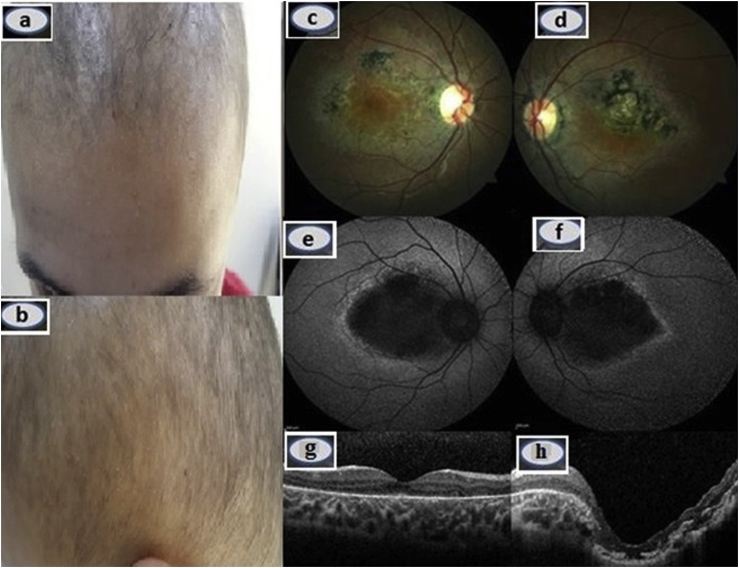
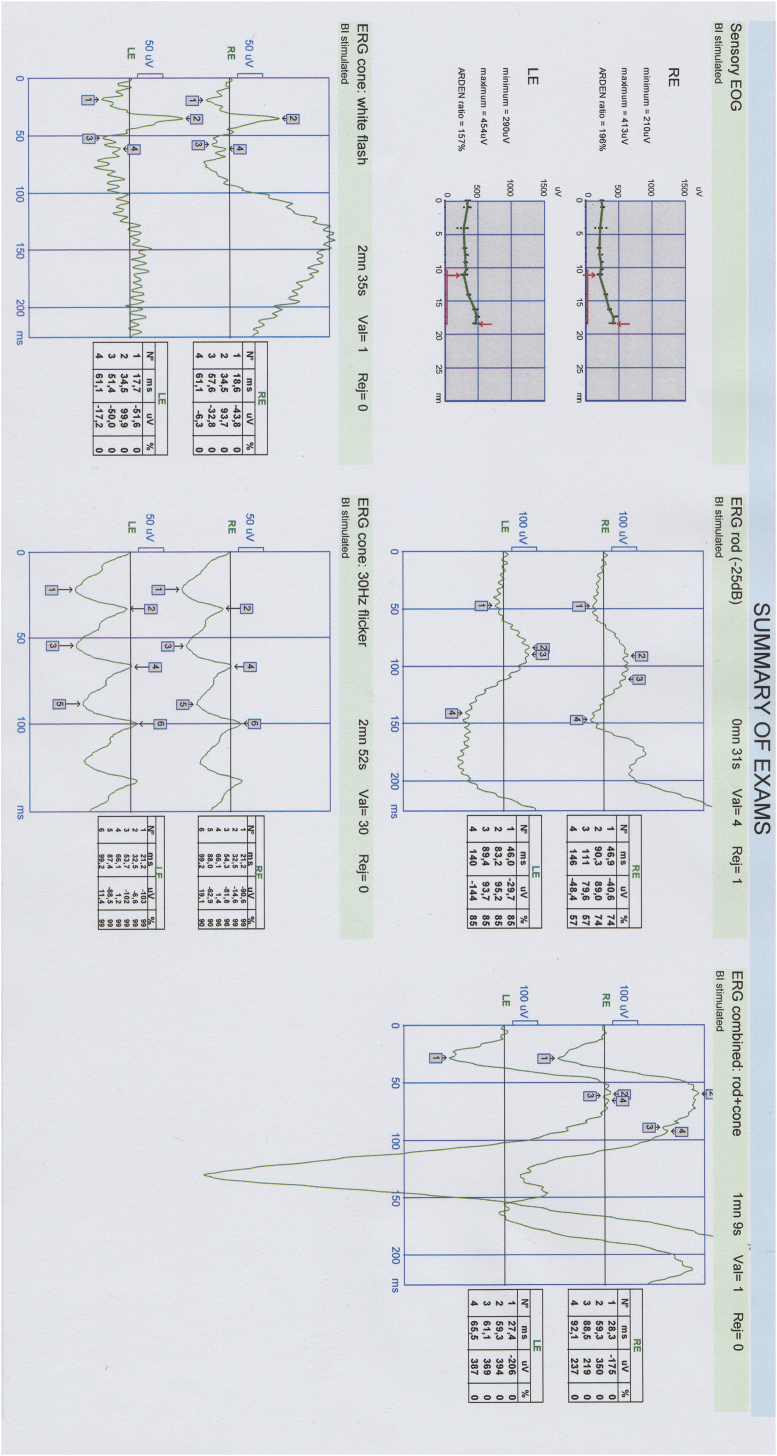
A 13-year-old girl with a clinical diagnosis of congenital hypotrichosis was referred to us with bilateral progressive visual deterioration. The visual symptoms commenced when she was 8 years old. Her parents, of Turkish origin, were not consanguineous and she had a healthy brother. Neither her parents nor the brother had hypotrichosis or any visual complaint. On physical examination, she had short, straight sparse and slow growing hair without erythema or scales (Fig. 1a and b). Hair pull test results and scalp skin texture were normal. The eyebrows and eyelashes were normal. No associated abnormality in her teeth, nails, or limbs was noted. Otherwise, her skin was normal. Complete blood count and ferritin, iron, transferrin, vitamin B12, and thyroid hormone levels were within normal ranges. On eye examination, eye movements and pupillary reflexes were normal and no afferent pupillary defect was present. Best-corrected visual acuity was 9/10 in OD and 1/10 in OS. Cycloplegic refraction was +0.50 diopters OU. Axial eye length was measured with an IOL Master (ver. 3.02; Carl Zeiss, Meditec, Jena, Germany) as 23.49 mm in OD and 24.25 mm in OS. Slit-lamp examination was unremarkable OU. Intraocular pressure was 16 mmHg bilaterally. Fundus examination revealed ring-shaped atrophy of the RPE with patchy intraretinal pigment clumping at the posterior pole bilaterally. However, foveola seemed to be relatively preserved in OD. Atrophy of the RPE and choriocapillaris involved the foveal region of the left eye. The affected posterior pole and healthy looking equatorial retina were well-demarcated bilaterally (Fig. 1c and d). Autofluorescence imaging showed a symmetrical wedge-shaped hypoautofluorescent area, corresponding to the RPE atrophy, which was surrounded by a hyperautofluorescent rim (Fig. 1e and f). Optical coherence tomography (OCT) demonstrated disruption of the photoreceptor and inner segment/outer segment junction, retinal thinning in OU, and a posterior staphyloma-like appearance in OS (Fig. 1g and h). Electrophysiological tests were performed using a MetroVision system (MetroVision, Perenchies, France). The electrooculogram (EOG) was within normal limits in OD but subnormal in OS. The rod electroretinogram (ERG) was considered subnormal OU. The standard-combined ERG, cone ERG, and flicker ERG were within normal limits OU (Fig. 2).
Fig. 1.
a, b Hypotrichosis associated with sparse and short hair. c, d Color fundus picture of the OD c and OS d indicating the central ring-shaped retinal pigment epithelium (RPE) atrophy and clearly demarcated healthy retina and affected posterior pole, and e, f showing the symmetrical wedge-shaped hypoautofluorescent area surrounded by a hyperautofluorescent rim. g, h Optical coherence tomography appearance. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Electrophysiological tests.
1.2. Genotyping and mutation analysis
Genomic DNA was isolated from a peripheral blood sample using a salting-out method. All of the coding region, along with flanking intron regions, of the CDH3 gene was amplified by polymerase chain reaction (PCR; primer sequences are available on request). For sequencing, the PCR products were purified using the ExoSAP-IT (GE Healthcare Bio-Sciences, Piscataway, NJ, USA), following the manufacturer's protocol. Sequence reactions were run on an ABI Prism 3130xl DNA Sequencer and analyzed with the supplied sequencing analysis software (ver. 5.4; Applied Biosystems, Foster City, CA, USA). DNA sequencing analysis detected a novel homozygous deletion (c.447_467del (p.149_156del)) in exon 5 of the patient. This non-frameshift deletion was predicted as disease-causing by MutationTaster. Both healthy parents and the older brother were heterozygous for the mutation, consistent with an autosomal-recessive mode of inheritance. This mutation has not been reported before in HJMD syndrome.
2. Discussion
Sprecher et al.4 showed the first mutation in the CDH3 gene causing HJMD in a Druze-origin family. A few years later, Kjaer et al.5 reported that mutations in the CDH3 gene also caused ectodermal dysplasia, ectrodactyly, and macular dystrophy (EEM syndrome; OMIM 225280). These two overlapping syndromes have common features, such as hypotrichosis and macular dystrophy. It was thought that EEM syndrome differed from HJMD by the presence of limb anomalies. However, recent reports and genetic analyses showed that limb anomalies may be present in both syndromes.6 Basel-Vanagaite et al.2 suggested that these syndromes represented a continuous phenotypic spectrum related to mutations in the CDH3 gene and classified them as CDH3-related syndromes, rather than different genetic syndromes.
Moreover, patients with same genotype in CDH3-related syndromes may have different clinical manifestations. The same mutation (c.829del: p. G277fs) in exon 7 was found in a Turkish HJMD family and a Brazilian EEM family in two separate studies by Indelman6 and Kjaer et al.5 Hair anomalies and retinal pigmentary alterations were present in both families but limb defects were observed only in the EEM family. Thus, Kjaer et al.5 suggested that HJMD and EEM represented phenotypic heterogeneity within the same syndrome. This considerable phenotypic variation for the same mutation may also point toward other genetic factors.
The CDH3 gene consists of 16 exons, spanning ∼55 kilobases of genomic DNA, and is localized on the long arm of chromosome 16. The CDH3 protein is a member of the P-cadherin protein family and conforms to the general structure of the ‘classic’ cadherins, a group of molecules expressed in a tissue-specific manner that form a major part of adherence junctions in most epithelial tissues. Classical cadherins are composed of five extracellular domains (ECs), one transmembranal domain (TM), and one intracytoplasmic domain (IC)2, 9, 10, 11, 12, 13, 14, 15 The first extracellular domain (EC1), encoded by the DNA sequences of exons 4 and 5, is important for hemophilic adhesion and contains conserved tryptophan residues that are responsible for trans-cadherin binding. To date, 20 mutations – 7 deletions,4, 7, 10, 17, 18 4 missense,7, 9, 10, 12 4 splice-site,13, 14, 15 3 nonsense,11, 16, 18 and 2 compound heterozygous6 – have been reported in the CDH3 gene in patients with HJMD. Table 1 summarizes the reported CDH3 gene mutations in HJMD. In the present study, we have identified a novel homozygous for a non-frameshift deletion mutation (c.447_467del (p.149_156del)) in exon 5, which affects the EC1 of P-cadherin. This mutation is predicted to result in a markedly altered structure and function of P-cadherin, which likely disrupts the mechanism of cell-cell adhesion.
Table 1.
CDH3 mutations and clinical features in previously reported patients with hypotrichosis with juvenile macular dystrophy.
| References | Origin | Visual acuity (OD/OS) | Skalp hypotrichosis | Macular pigment degeneration | Additional clinical findings | CDH3 mutation types | |
|---|---|---|---|---|---|---|---|
| Sprecher et al.,4 2001 | Israeli | NR | + | + | NR | c.981del (p.M327fs) | Homozygous frameshift deletion |
| Indelman et al.,6, 9, 10, 11 2002 | Israeli | NR | + | + | NR | c.1508G > A (p.R503H) | Homozygous missense |
| 2003 | French | NR | + | + | Atopic dermatitis | c.503T > A (p.L168X) c.2112del (p.G706fs) | Heterozygous Stopgain and heterozygous frameshift deletion |
| 2003 | Turkish | NR | + | + | Keratosis pilaris | c.829del (p.G277fs) | Homozygous frameshift deletion |
| 2003 | Israeli | NR | + | + | Centrofacial lentiginosis | c.1508G > A (p.R503H) | Homozygous missense |
| 2003 | Israeli | NR | + | + | NR | c.462del (p.E155fs) | Homozygous frameshift deletion |
| 2005 | Arab | OD:20/28, OS: 20/33 | + | + | NR | c.1845T > G (p.Y615X) | Homozygous Stopgain |
| 2007 | English | OD: 6/36, OS: 6/5 | + | + | Limb abnormalities | c. IVS2+1G > A c.1510G > A (p.E504K) | Heterozygous Splice site and Heterozygous missense |
| 2007 | American | NR | + | + | Discolored primary teeth, nail dystrophy | c.661C > T (p.R221X) c.1724A > G (p.H575R) | heterozygous Stopgain and heterozygous frameshift deletion |
| Bergman et al.,12 2004 | Arab-Israeli | NR | + | NR | Centrofacial lentiginosis | c.1508G > A (p.R503H) | Homozygous missense |
| Liebu et al.,7 2006 | Israeli | NR | + | + | NR | c.981del (p.M327fs) | Homozygous frameshift deletion |
| 2006 | Israeli | NR | + | + | NR | c.1508G > A (p.R503H) | Homozygous missense |
| Jelani et al.,13 2009 | Pakistani | NR | + | + | NR | c.IVS10-1G > T | Homozygous Splice site |
| Hassan et al.,14 2010 | Pakistani | NR | + | + | NR | c.IVS10-1G > A | Homozygous Splice site |
| Shimomura et al.,15 2010 | Pakistani | NR | + | NR | NR | c.IVS12-2A > G | Homozygous Splice site |
| 2010 | Pakistani | NR | + | NR | NR | c.IVS10-1G > T | Homozygous Splice site |
| Avitan-Hersh.,16 2012 | Arab | NR | + | + | NR | c.747C > A (p.Y249X) | Homozygous Stopgain |
| Halford et al.,17 2012 | NR | OD: 6/760, OS: 6/96 | + | + | NR | Gross Deletion 8815bp including exons 12–13 | Homozygous deletion |
| Khan et al.,18 2016 | Arab | OD: 20/60, OS: 20/60 | + | + | Slow nail growth | c.307C > T (p.R103X) | Homozygous Stopgain |
| 2016 | Arab | OD: 3/200, OS: 3/200 | + | + | NR | c.307C > T (p.R103X) | Homozygous Stopgain |
| Present study | Turkish | OD: 0.9, OS: 0.1 | + | + | – | c.447_467del (p.149_156del) | Homozygous nonframeshift deletion |
Abbreviation: NR, not reported.
In a typical HJMD family, family members may have different phenotypes and present with various hair morphologies, skin changes, and eye involvement10 Furthermore, Indelman et al.10 reported significant inter- and intra-familial discrepancies in a series of families of various ethnic origins. In their study, even among members of the same family, the hair, skin, and retinal phenotypes varied widely. They reported an intriguing case of intra-familial phenotypic heterogeneity where two of three affected individuals in a family displayed centrofacial lentiginosis. They also reported three affected girls, aged 9, 11, and 13 years, in a family where the parents were healthy and of white, northern American descent. Only the eldest child had discolored primary teeth and nail dystrophy. In light of their observation, they suggested that variation in the phenotype was not correlated with the genotype.
Generally, HJMD patients have similar fundus changes, including ring-like retino-choroidal atrophy with patchy intraretinal pigment clumping at the posterior pole bilaterally.4, 6, 7, 8, 9, 10, 11, 13, 14, 16, 17, 18 However, Mason et al.8 described a HJMD patient with bull's eye maculopathy. Liebu et al.7 also pointed out that some patients with HJMD might have different fundus appearances, such as diffuse hypopigmentation associated with coarse granular pigmentation and white-yellowish flecks in the macular area and beyond. They demonstrated that the type and extent of fundus abnormality might vary between patients with different disease entities despite having the same genetic mutation. A macular hole was described by Khan et al.18 In HJMD patients, no phenotype-genotype correlation has been established for hair and retinal changes between the type of mutation and its location along the CDH3 gene. These studies suggest that the allelic mutation may not be the only factor that determines the clinical status. Given this complex genotype-phenotype correlation of CDH3 mutations, more research is required.
In all previous reports on patients with HJMD, reduced visual acuity was the presenting ocular symptom, as in our case. Visual deterioration commences in the first decade of life6, 7, 11, 18 However, Halford et al.17 described two patients who experienced visual loss at the age of 17 years. The extent of vision loss varies, and is difficult to predict. Visual acuity usually declines to 10/32 or worse.17, 18 In the present case, the visual symptoms commenced when our female patient was 8 years old and worsened over the next 2 years. At an age of 10 years, her visual acuity was 10/10 in the right and 2/10 in the left eye. However, at her most recent visit, the visual acuity was 9/10 in the right and 1/10 in the left eye. Disruption of the photoreceptor-inner/outer segment junction was more prominent in the left than the right eye. The left eye also exhibited a posterior staphyloma-like appearance. Therefore, the visual loss was more severe. Many studies have included visual acuity data; the losses were usually symmetrical.11, 17, 18 However, asymmetric involvement was reported by Indelman et al.6 The visual acuity was 6/36 in the right and 6/5 in the left eye of one patient. The extent of asymmetry in our case was similar.
3. Conclusions
We describe a novel homozygous mutation (c.447_467del (p.149_156del)) in the CDH3 gene in a non-consanguineous Turkish family. No significant phenotypic difference was observed between our case and previously described patients with CDH3 mutations from different parts of the world. As HJMD is a rare clinical entity, pediatricians, dermatologists, and ophthalmologists in particular should be aware of the clinical spectrum of this disease. Sparse hair in childhood, with or without limb anomalies, should alert clinicians to request an ophthalmological evaluation to assess possible retinal abnormalities.
Patient consent
Patient's legal guardian consented in writing to publication of the case.
Funding
None.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authorship
All authors contributed significantly to the creation of this manuscript, each fulfilled criteria as established by the ICMJE.
Acknowledgements
The authors thank Textcheck for editing the manusciprt.
References
- 1.Wagner H. Makulaaffektion vergesellschaftet mit Haarabnormalitat von anugotypus, beide vielleicht angeboren bei zwei Geschwistern. Graefes Arch Klin Exp Ophthalmol. 1935;134:71. [Google Scholar]
- 2.Basel Vanagaite L., Pasmanik M., Lurie R., Yeheskel A., Kjaer K.W. CDH3 -related syndromes: report on a new mutation and overview of the genotype-phenotype correlations. Mol Syndromol. 2010;1:223–230. doi: 10.1159/000327156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duverger O., Morasso M.I. To grow or not to grow: hair morphogenesis and human genetic hair disorders. Semin Cell Dev Biol. 2014;25–26:22–33. doi: 10.1016/j.semcdb.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sprecher E., Bergman R., Richard G. Hypotrichosis with juvenile macular dystrophy is caused by a mutation in CDH3, encoding P-cadherin. Nat Genet. 2001;29:134–136. doi: 10.1038/ng716. [DOI] [PubMed] [Google Scholar]
- 5.Kjaer K.W., Hansen L., Schwabe G.C. Distinct CDH3 mutations cause ectodermal dysplasia, ectrodactyly, macular dystrophy (EEM syndrome) J MedGenet. 2005;42:292–298. doi: 10.1136/jmg.2004.027821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Indelman M., Eason J., Hummel M. Novel CDH3 mutations in hypotrichosis with juvenile macular dystrophy. Clin Exp Dermatol. 2007;32:191–196. doi: 10.1111/j.1365-2230.2006.02335.x. [DOI] [PubMed] [Google Scholar]
- 7.Leibu R., Jermans A., Hatim G. Hypotrichosis with juvenile macular dystrophy: clinical and electrophysiological assessment of visual function. Ophthalmology. 2006;113:841–847. doi: 10.1016/j.ophtha.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 8.Mason J.O., Patel S.A. A case of hypotrichosis with juvenile macular dystrophy. Retin Cases Brief Rep. 2015;9:164–167. doi: 10.1097/ICB.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 9.Indelman M., Bergman R., Lurie R. A missense mutation in CDH3, encoding P-cadherin, causes hypotrichosis with juvenile macular dystrophy. J Invest Dermatol. 2002;119:1210–1213. doi: 10.1046/j.1523-1747.2002.19528.x. [DOI] [PubMed] [Google Scholar]
- 10.Indelman M., Hamel C.P., Bergman R. Phenotypic diversity and mutation spectrum in hypotrichosis with juvenile macular dystrophy. J Invest Dermatol. 2003;121:1217–1220. doi: 10.1046/j.1523-1747.2003.12550_1.x. [DOI] [PubMed] [Google Scholar]
- 11.Indelman M., Leibu R., Jammal A., Bergman R., Sprecher E. Molecular basis of hypotrichosis with juvenile macular dystrophy in two siblings. Br J Dermatol. 2005;153:635–638. doi: 10.1111/j.1365-2133.2005.06734.x. [DOI] [PubMed] [Google Scholar]
- 12.Bergman R., Sapir M., Sprecher E. Histopathology of hypotrichosis with juvenile macular dystrophy. Am J Dermatopathol. 2004;26:205–209. doi: 10.1097/00000372-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Jelani M., Salman Chishti M., Ahmad W. A novel splice-site mutation in the CDH3 gene in hypotrichosis with juvenile macular dystrophy. Clin Exp Dermatol. 2009;34:68–73. doi: 10.1111/j.1365-2230.2008.02933.x. [DOI] [PubMed] [Google Scholar]
- 14.Kamran ul Hassan Naqvi S., Azeem Z., Ahmad W. A novel splice-acceptor site mutation in CDH3 gene in a consanguineous family exhibiting hypotrichosis with juvenile macular dystrophy. Arch Dermatol Res. 2010;302:701–703. doi: 10.1007/s00403-010-1035-6. [DOI] [PubMed] [Google Scholar]
- 15.Shimomura Y., Wajid M., Kurban M., Christiano A.M. Splice site mutations in the P-cadherin gene underlie hypotrichosis with juvenile macular dystrophy. Dermatology. 2010;220:208–212. doi: 10.1159/000275673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avitan Hersh E., Indelman M., Khamaysi Z., Leibu R., Bergman R. A novel nonsense CDH3 mutation in hypotrichosis with juvenile macular dystrophy. Int J Dermatol. 2012;51:325–327. doi: 10.1111/j.1365-4632.2011.04973.x. [DOI] [PubMed] [Google Scholar]
- 17.Halford S., Holt R., Németh A.H., Downes S.M. Homozygous deletion in CDH3 and hypotrichosis with juvenile macular dystrophy. Arch Ophthalmol. 2012;130:1490–1492. doi: 10.1001/archophthalmol.2012.708. [DOI] [PubMed] [Google Scholar]
- 18.Khan A.O., Bolz H.J. Phenotypic observations in “hypotrichosis with juvenile macular dystrophy” (recessive CDH3 mutations) Ophthalmic Genet. 2016;37:301–306. doi: 10.3109/13816810.2015.1071411. [DOI] [PubMed] [Google Scholar]