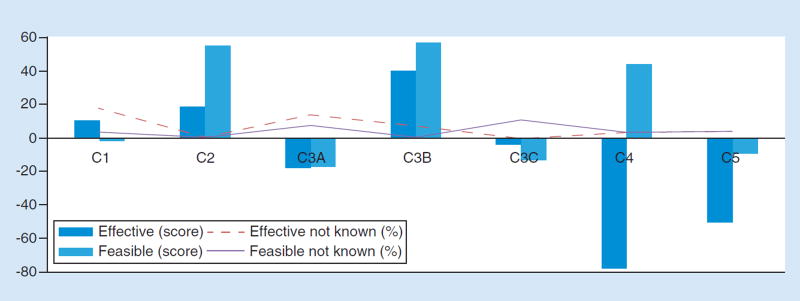
Figure 4. Effectiveness and feasibility scores for solutions to mitigating risk to patients from NGS-based LDTs.
(C1) Use FDA oversight as a mechanism to address the risks to patients from next-generation sequencing (NGS)-based laboratory-developed tests (LDTs). (C2) Use laboratory accreditation and proficiency testing by professional societies as a mechanism to address the risks to patients from NGS-based LDTs. (C3A) Use payers to address certain risks to patients from NGS-based LDTs, specifically, restrict reimbursement. (C3B) Use payers to address certain risks to patients from NGS-based LDTs, specifically, to restrict payment to accredited laboratories. (C3C) Use payers to address certain risks to patients from NGS-based LDTs, specifically, condition coverage on consultation with a genetic counselor. (C4) Rely on self-regulation to address the clinical risks to patients from NGS-based LDTs. (C5) Rely on state regulation to address the risks to patients from NGS-based tests.
LDT: Laboratory-developed test; NGS: Next-generation sequencing.