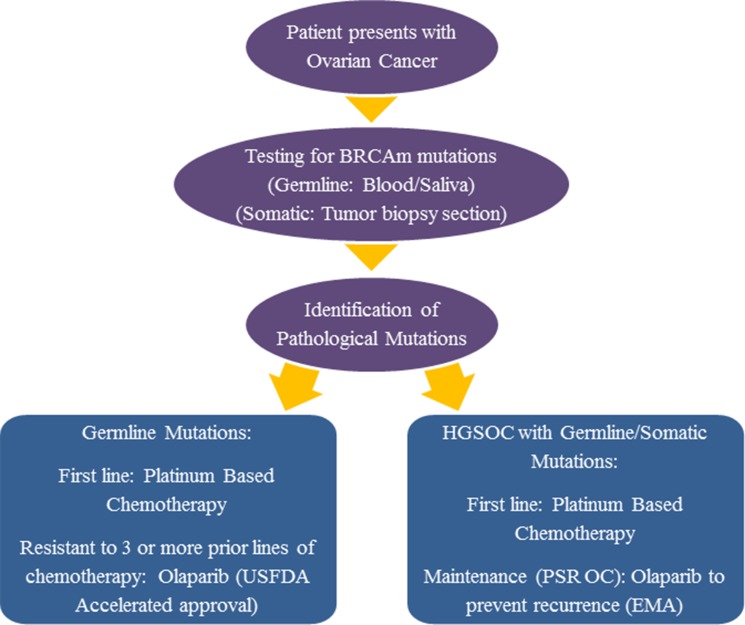
Figure 1. Incorporation of olaparib into the treatment regimen for BRCAm OC: USFDA versus EMA approval.
Outline of the testing and treatment procedure for patients presenting to the clinic with Ovarian Cancer. A comparison of the approval status for PARPi (Olaparib) in Europe versus America has been described. While the FDA has approved the use of Olaparib in patients with OC that failed to respond to three or more lines of chemotherapy, the EMA has approved Olaparib as maintenance treatment for PSR OC.