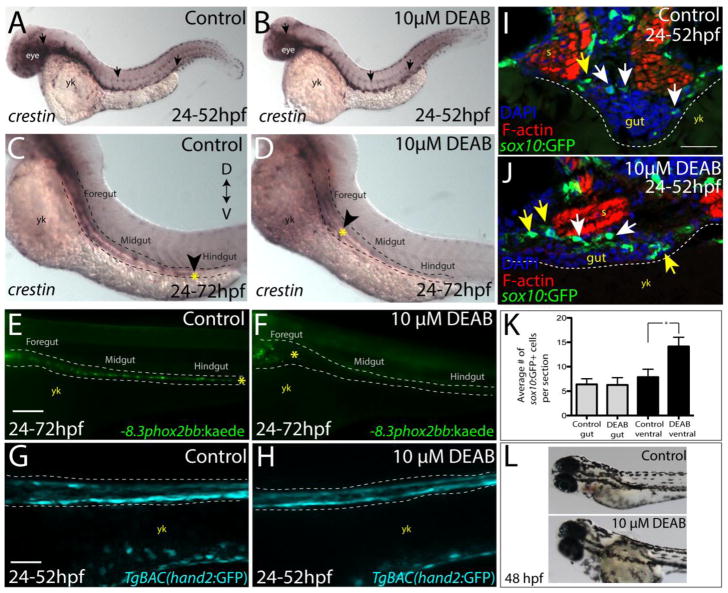
Figure 3. Temporal loss of RA stalls migration of enteric neural crest within the foregut without affecting enteric neural crest cell numbers.
(A–B) Whole-mount in situ hybridization against crestin in (A) control and (B) DEAB treated larvae at 52 hpf.
(C–D) Whole-mount in situ hybridization against crestin in control and DEAB treated larvae at 72 hpf reveals that enteric neural crest are delayed along the foregut, when compared with controls. Arrows and yellow asterisk marks caudal end of enteric neural crest migratory front along the gut. yk-yolk
(E–F) Live images of −8.3phox2bb:Kaede (E) control and (F) DEAB treated larvae at 72 hpf reveals that enteric progenitors are delayed in migration along the foregut, when compared with control cells along the hindgut. yk-yolk
(G–H) Live confocal projection images of hand2:GFP in (G) control and (H) DEAB treated larvae at 52 hpf reveals the presence of gut mesenchyme laterally along the gut.
(I–J) Transverse cryosections show that sox10:GFP+ cells located near the foregut in (I) control and (J) DEAB treated larvae. When compared with control sections, DEAB treated larvae exhibit increased numbers of neural crest in the mesenchyme surrounding the gut (yellow arrows), while number of neural crest in direct gut contact (white arrows) are not affected. s-somite, yk-yolk
(K) Bar graph depicting the average number of sox10:GFP+ neural crest with direct gut contact or in the surrounding ventral mesenchyme near the gut in control and DEAB treated larvae. n=9 embryos for each condition. Error bars indicate +/− S.E.M. *, p < .05 with Student’s t-test.
(L) Bright field images of a control (top) and DEAB treated (bottom) larval fish at 48 hpf to reveal the distribution of melanophores.
Scale bars in panels E–H, 100 μM; scale bar in panel I–J, 30 μM.