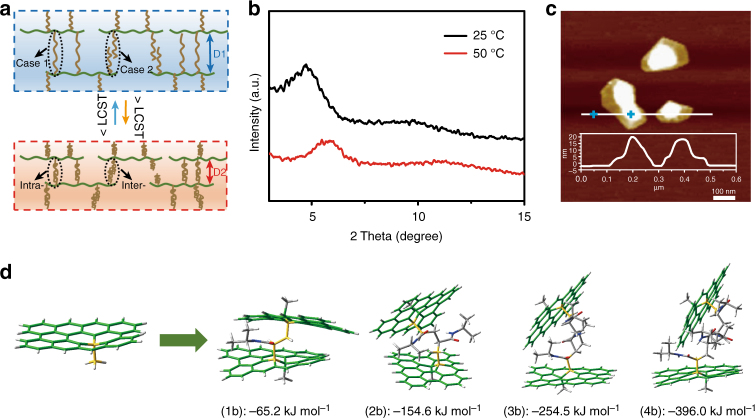
Fig. 4.
Mechanism of the negative temperature-response behavior of P-GOMs. a Schematic diagram of the channel size change of P-GOMs from D1 to D2 caused by the swelling or shrinking of PNIPAM chains covalent bound to GO sheets. Case 1: PNIPAM chains grow from one GO sheet and terminate at another GO sheet. Intramolecular hydrogen bonding is formed when the temperature is above its LCST. Case 2: PNIPAM chains grow from one GO sheet and terminate freely. Intermolecular hydrogen bonding is formed when the temperature is above its LCST. b The XRD patterns of P-GOMs at 25 °C and 50 °C. The right shift of the diffraction peak indicates the smaller lamellar distance at 50 °C than 25 °C. c The AFM image of P-GO. The height of cross-sectional profile proves that the P-GO sheets would like to stack together at 50 °C. d Optimized geometries of [C36H16-CH3]• and the representative products of reactions (1b) C36H16-CH3-NIPAM-CH3-C36H16, (2b) C36H16-CH3-(NIPAM)2-CH3-C36H16, (3b) C36H16-CH3-(NIPAM)3-CH3-C36H16 and (4b) C36H16-CH3-(NIPAM)4-CH3-C36H16. Others are shown in Supplementary Fig. 16. The graphene sheet is shown in green tubes, the PNIPAM as tubes (atomic color scheme: C, gray; N, blue; O, red; H, white). The newly formed bond is highlighted in yellow