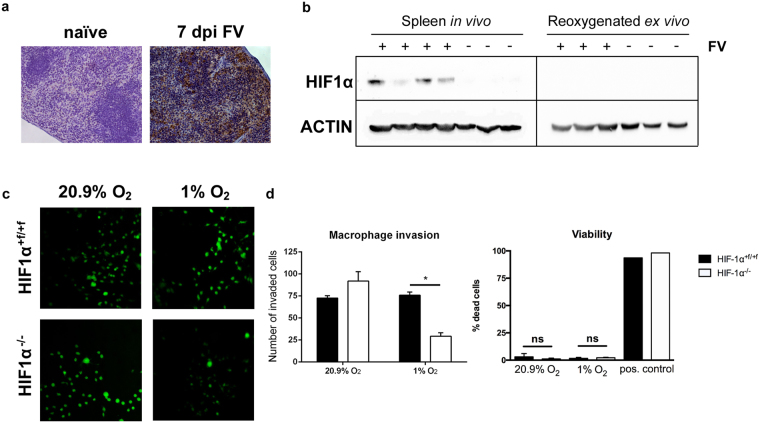
Figure 3.
Loss of hypoxia-inducible factor 1α from macrophages impairs invasion capabilities under hypoxic conditions. Sections of spleen tissue from naïve and Friend virus (FV)-infected mice (7 days after infection [dpi]) were stained with 3,3′- diaminobenzidine (DAB) pimonidazole (brown) and then counterstained with haematoxylin (a, blue). Spleens of naïve and FV-infected mice (7 dpi) were removed, and proteins were directly isolated in a hypoxic workstation under hypoxic conditions (1% O2) so that rapid degradation of hypoxia-inducible factor (HIF) protein could be avoided. One part of the spleen was removed from the workstation and exposed to atmospheric conditions (20.9% O2) for 10 minutes for reoxygenation before protein isolation. Subsequently, Western blot analysis was performed with specific antibodies for HIF-1α and ACTIN (b). An inverted invasion assay was performed to compare 3D invasion of bone marrow–derived macrophages (BMDMs) from naïve wild-type (WT) and HIF-1α knockout (KO) mice under normoxic and hypoxic conditions. BMDMs were allowed to invade a Matrigel/fibronectin gel for 72 hours. Subsequently, cells were stained with calcein acetoxymethyl (AM) and visualised by confocal microscopy (c; original magnification, 200×). Three randomly chosen fields per well were recorded, and the number of invaded BMDMs was determined. For viability testing, dead BMDMs were stained with DAPI, and stained cells were counted manually in relation to the total number of cells in the field. Positive controls were treated with 0.1% Triton X-100. (d). Data were analysed with Student’s t-test (mean + SEM). n = 3–4. *P = 0.05.