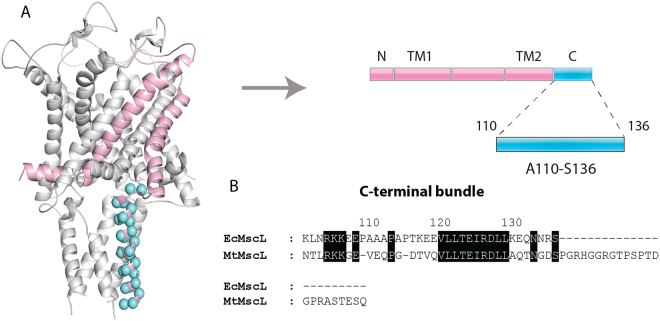
Figure 1.
3D homology model of the closed EcMscL channel. (A) Single MscL subunit (rose pink) showing amino acid residues comprising the A110-S136 segment of the C-terminal bundle encompassing 27 residues subjected to cysteine scanning mutagenesis (blue spheres). The single MscL monomer is represented as a part of the channel pentamer according to the 3D homology model of EcMscL as described in the methods6 (left). Linear representation of the membrane topology of EcMscL (right). The N- and C-terminal domains, transmembrane helices TM1 and TM2 and the periplasmic and cytoplasmic loops are represented by rose pink and blue rectangles, respectively. (B) Sequence alignment of the C-terminal bundle of EcMscL and MtMscL. Identical residues are highlighted in black. The numerical equivalence between the residues shown in the figure was generated using a ClustalW pairwise sequence alignment between the two helical bundle sequences.