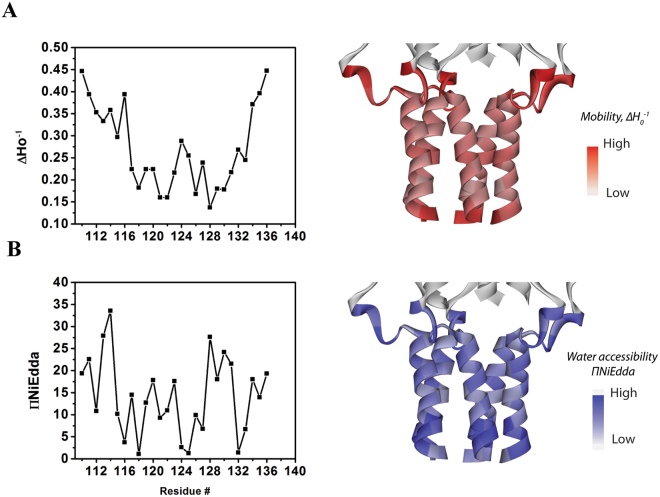
Figure 2.
Residue-specific environmental parameter profiles obtained for the A110–S136 segment of the C-terminal bundle. (A) Mobility parameter ΔH0 –1 indicates the greatest mobility for the first seven residues A110–T116 and three last residues N134–S136, which suggests these residues form a free loop and have no defined secondary structure, respectively (left). A periodicity in the mobility of the spin label from T116 through to E131 residues is consistent with the observed α-helical structure of this portion of the C-terminal bundle (right) in the crystal structure of MtMscL (D108–Q123 in MtMscL), which is not considered to be in the fully open state but an expanded state (Li, Jie, et al., 2015, PNAS). Importantly however, MaMscL has a vastly different C-terminus compared to the EcMscL in terms of the number of helices, sequence and structure. Nevertheless, the transmembrane helices share high similarity in sequence and gating mechanism6. (B) NiEdda accessibility parameter ΠNiEdda shows high accessibility of most helical bundle residues to NiEdda and thus to the aqueous compartment consistent with the cytoplasmic location of the bundle (left). The profile of ΠNiEdda is also roughly correlates to the profile of the mobility parameter ΔH0 −1 shown in (A). Accessibility to NiEdda indicated in blue on the crystal structure of the helical bundle (right).