Figure 2. Important Afferent and Efferent Insular Pathways in Migraine.
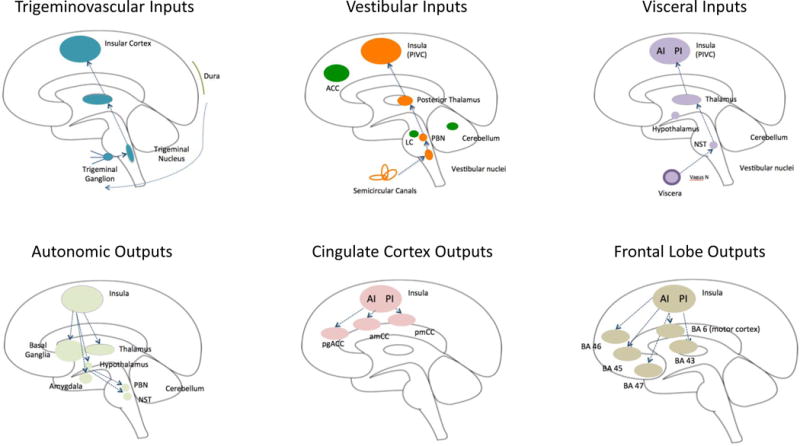
A: Afferent inputs (top row):
Trigeminovascular: The trigeminal system includes afferent inputs from the 3 major divisions of the trigeminal nerve (ophthalmic, maxillary and mandibular) that send their inputs to the trigeminal nuclear complex (Willis and Westlund, 1997). Projections from here are primarily to the thalamus and to the amygdala and hypothalamus through trigeminothalamic (Matsushita et al., 1982), trigemino-parabrachial-amygdala (Bernard and Besson, 1990), and trigeminohypothalamic (Malick and Burstein, 1998) tracts. Projections from these regions to the insula have been described for each primary projection complex (thalamo-insula (Mufson and Mesulam, 1984); hypothalamo-insula (Saper, 2000) and amygdalo-insula (Kevetter and Winans, 1981). As noted in the text some of these connections have been reported in functional imaging studies in migraine patients (amygdala-insular connections (Hadjikhani et al., 2013); see Figure 3B).
Vestibular: Vestibular inputs originate from the semicircular canals in the inner ear and project to the vestibular nuclei in the brainstem. The afferent pathway to the insula includes the parabrachial nuclei, posterior thalamus to the parieto-insula vestibular cortex (Brandt and Dieterich, 1999). These pathways are reviewed elsewhere (Angelaki and Cullen, 2008).
Visceral: Visceral inputs to the insula include pathways from the viscera (e.g., vagus nerve) to the nucleus of the solitary tract (NTS) (McDougal et al., 2011). Projections from the thalamus to the posterior insula relay visceral sensory information (Cechetto, 2014).
B: Efferent outputs (bottom row):
Autonomic: The insula is involved in cortical regulation of autonomic control (Nagai et al., 2010) (Jones, 2011). Efferents from the insula connect with multiple subcortical (basal ganglia, thalamus, and hypothalamus) and brainstem regions (e.g., parabrachial nucleus, nucleus of the solitary tract) (Beissner et al., 2013; Benarroch, 1993).
Cingulate Cortex: Connections between the insula and cingulate cortex are part of a number of networks. It has been postulated that the interactions between the insula and the cingulate are involved in ‘integrating interoceptive information with emotional salience’ (pACC/aMCC) or involved in ‘environmental monitoring, response selection, and skeletomotor body orientation’ (MCC) (Taylor et al., 2009). Pathways that are part of a circuit for processes such as empathy for pain (Engen and Singer, 2013; Singer et al., 2009; Singer et al., 2004) or the initiation of goal-directed behaviors (Devinsky et al., 1995).
Frontal Lobe: Pathways projecting from the insula to the frontal and motor cortex. The anterior insula connects to Brodmann Areas (BA) 45, 46, 47 and 6 (motor cortex), while the posterior insula has connections with 43. These connections are implicated in cognitive processing, speech, memory encoding and recognition (Ramnani and Owen, 2004; Wood and Grafman, 2003).
Key: ACC = Anterior Cingulate Cortex, PIVC = Parieto-Insular Vestibular Cortex; LC = Locus Coeruleus; PBN = Parabrachial Nucleus; AI = Anterior Insula; PI = Posterior Insula; NST = Solitary Nucleus; pgACC = Pregenual Anterior Cingulate Cortex; amCC = Anterior Midcingulate Cortex; pmCC = Posterior Midcingulate Cortex; BA = Brodmann Area.
