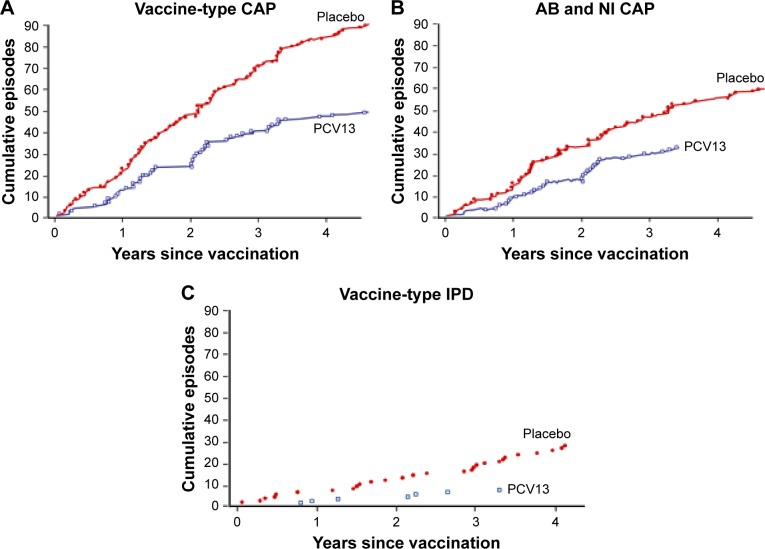
Figure 4.
Efficacy of PCV13 in older adults: results of the CAPITA study.
Notes: CAPITA was a randomized, double-blind, placebo-controlled trial that evaluated the efficacy of 13-valent polysaccharide conjugate vaccine (PCV13) versus placebo in 84,496 adults ≥65 years of age. The primary objective was vaccine efficacy in preventing a first episode of vaccine-type pneumococcal community-acquired pneumonia (CAP). A secondary objective was vaccine efficacy in preventing a first episode of vaccine-type abacteremic (AB) and noninvasive (NI) pneumococcal CAP. The figure illustrates post hoc analyses of the cumulative episodes of primary and secondary efficacy end points in the per-protocol population. (A) Cumulative first episodes of vaccine-type CAP from vaccination to 5-year follow-up. Vaccine efficacy was 45.6% (95.2% CI: 21.8%–62.5%, P<0.001). (B) Cumulative first episodes of vaccine-type AB and NI CAP from vaccination to 5-year follow-up. Vaccine efficacy was 45% (95.2% CI: 14.2%–65.3%, P=0.007). (C) Cumulative first episodes of vaccine-type invasive pneumococcal disease (IPD) from vaccination to 5-year follow-up. Vaccine efficacy was 75% (95% CI: 41.4%–90.8%, P<0.001). From N Engl J Med. Bonten et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. 372:1114–1125. Copyright © 2015 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.8
Abbreviation: CAPITA, Community-Acquired Pneumonia Immunization Trial in Adults.