Figure 2. Current and upcoming CFTR modulator clinical trials.
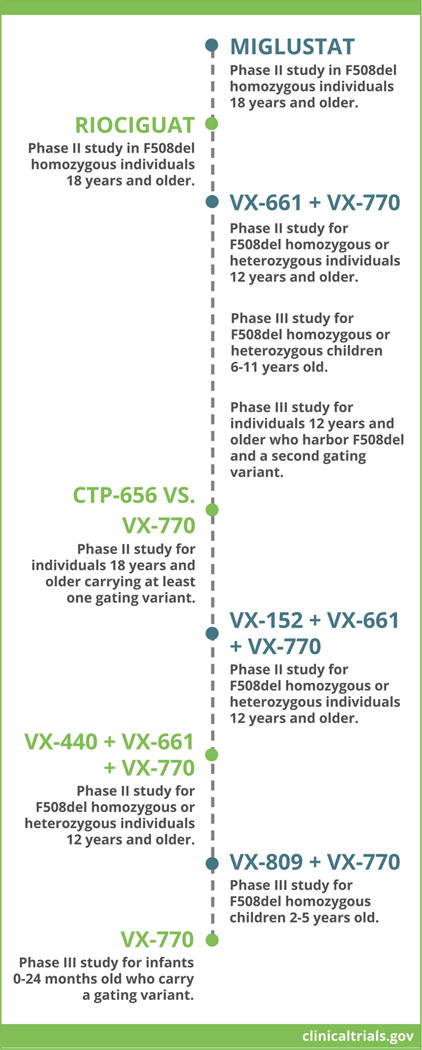
Examples of phase II or III clinical trials under enrollment in the United States and Europe are listed (see also www.clinicaltrials.gov). Various strategies outlined above intend to test safety, tolerability, and efficacy of CFTR modulators administered as monotherapy (e.g. Miglustat), combination therapy with two agents (e.g. VX-661 + VX-770), or combination therapy with three agents (e.g. VX-152 + VX-661 + VX-770). In the majority of cases, eligible patients must be homozygous or heterozygous for the most prevalent CFTR variant, F508del, or carry a gating or partial function defect.
