Figure 2. Comparison of pioglitazone and placebo treatment periods for the primary end-point of asthma quality of life questionnaire score and secondary end-points of asthma symptoms and control.
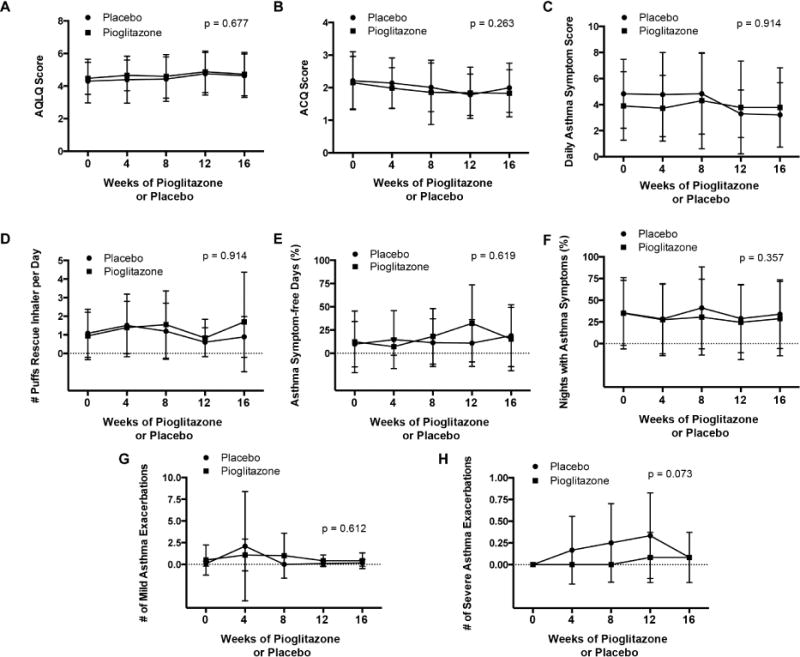
Panel A. Juniper asthma quality of life (AQLQ) score. Panel B. Juniper asthma control questionnaire (ACQ) score. Panel C. Daily asthma symptom score. Panel D. Number of puffs of rescue β2-agonist inhaler per day. Panel E. Asthma symptom-free days (%). Panel F. Nights with asthma symptoms (%). Panel G. Number of mild asthma exacerbations. Panel H. Number of severe asthma exacerbations. Data for daily asthma symptom score, rescue β2-agonist inhaler use, asthma symptom-free days, nights with asthma symptoms are averages for each period based upon the days that subjects reported data. P values are shown for differences between the pioglitazone and placebo treatment periods.
