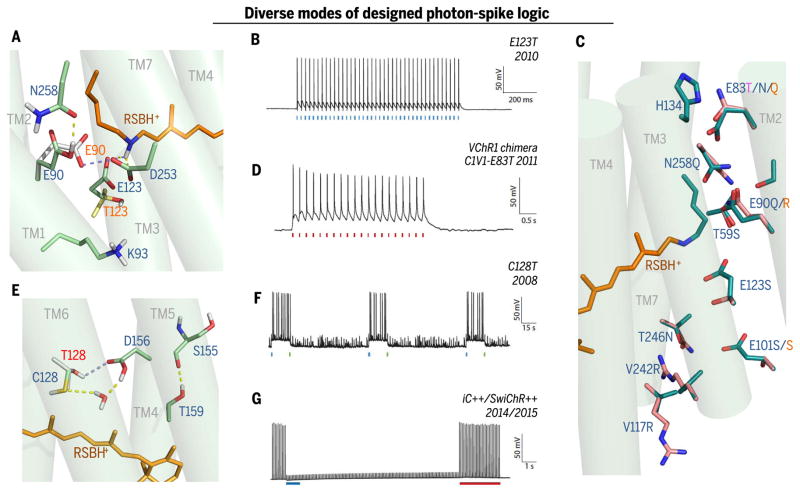
Fig. 4. Diverse modes of photon-spike transduction logic with underlying structural design.
(A) Snapshot of ChR active site (MD calculation from C1C2 structure 3UG9, with predicted repositioning of side chains resulting from Glu123 → Thr (E123T) mutation in red lettering; E123Tcauses inward flipping of Glu90 to compensate for the lost Glu123 counterion (43) of the RSBH+, additionally preventing inactivation. (B) Photon-spike transduction mode arising from ChETA mutation [ChR2-E123Tvariant (45)]: single blue flash–single spike coupling with high speed and high fidelity. Pore redesign implements faster closure after light-off, permitting rapid firing [e.g., 200-Hz trains in interneurons (45)]. (C) Pore residues (from C1C2 structure 3UG9) altered in spectral and selectivity variants. Modification of inner gate in red-activated C1V1-E83Tvariant (magenta letter–designated mutation; ChR2 numbering) (54). Selectivity variants are shown as original cation-conducting C1C2 pore residues and modifications to create the Cl−-selective iC++ [new pink side chains overlaid on original C1C2 green side chain positioning; blue letters denote iC++ mutations (62, 64)] or iChloC [orange letter–denoted mutations (93, 94)]. (D) Photon-spike transduction mode arising in Volvox-derived C1V1-E83T: single red flash–single spike coupling (54) with moderately high speed/fidelity; later Volvox derivative bReaChES exhibits faster responses with ChETA modification for accelerated channel closure (77) (not shown). (E) Snapshot of most likely structure of the DC-pair region in C1C2 (blue lettering) and the C128T (Cys128 → Thr) variant (red lettering) based on MD calculation (100) of restructured hydrogen-bonding network [yellow → blue dashed-line transition represents this SFO-mutation (52, 54) transition] and modified TM3-TM4 interaction (100), resulting in extension of open-state lifetime (52–54) and many-orders-of-magnitude-increased light sensitivity of expressing cells (42, 52, 54). (F) Photon-spike transduction mode arising from C128T (SFO) mutation is bistable, ultra–light-sensitive, two-color switchable, and excitatory [note blue light actuation and green light termination (52, 54)]. (G) Photon-spike transduction mode arising from adding the Cys128 SFO mutation (E) to Cl−-selective iC++ mutations (C) to create (64) SwiChR++ provides ultra–light sensitivity and is bistable, two-color switchable, and inhibitory under typical conditions (62, 64).