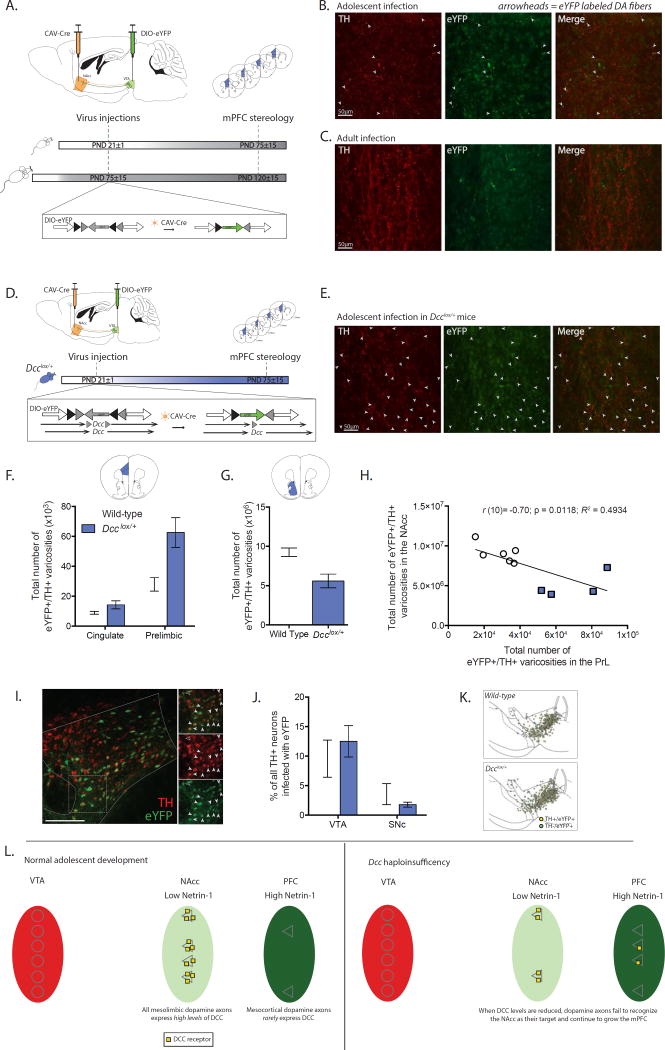
Figure 1. Mesocortical dopamine axons are still growing during adolescence; Dcc prevents the growth of mesolimbic dopamine axons to the mPFC by targeting them to the NAcc.
(A) Experimental strategy to label NAcc-projecting ventral tegmental area (VTA) dopamine neurons with eYFP from the start of adolescence (PND21±1) or during adulthood (PND75±15) using axon-initiated viral transduction. Six weeks later, at which point adolescent mice have reached adulthood, eYFP-positive dopamine axons in the mPFC were quantified. Inset: Viral recombination in midbrain neurons. (B) Representative micrographs of eYFP-positive dopamine fibers, indicated with arrowheads, which grew to the prelimbic subregion of the mPFC following viral injections in adolescent wild-type mice. (C) Representative micrographs from the prelimbic subregion of the mPFC of wild-type mice that received injections of the tracing viruses in adulthood. Although both TH-positive and eYFP-positive axons are present, eYFP-positive dopamine axons are exceedingly rare. (D) Infection strategy to combine axon-initiated viral transduction with conditional Dcc haploinsufficiency at the start of adolescence. Inset: In Dcclox/+ mice, the CAV-Cre virus also induces Dcc haploinsufficiency in labeled neurons. (E) Representative micrographs of eYFP-positive dopamine fibers, represented by arrowheads, in the prelimbic subregion of the mPFC of Dcclox/+ mice that received the viral injections at the start of adolescence. (F) Stereological quantification reveals that following adolescent injections (1) eYFP-positive dopamine varicosities are present in the mPFC in adult mice (i.e. dopamine axons continue to grow to from the NAcc into the mPFC during adolescence), and (2) the number of eYFP-positive dopamine varicosities is dramatically increased by Dcc haploinsufficiency (Two-way mixed-design ANOVA, main effect of genotype, F(1, 8) = 11.87, p = 0.0088; main effect of subregion, F(1, 8) = 38.03, p = 0.0003; genotype × subregion interaction, F(1, 8) = 7.262, p = 0.0273. n = 5 per group). (G) The number of eYFP-positive dopamine varicosities in the NAcc is dramatically reduced by Dcc haploinsufficiency (t(8) = 3.56, p = 0.0074) (H) Negative correlation between eYFP-positive dopamine varicosity number in the NAcc and PrL subregion of the mPFC. (I) Representative micrograph of eYFP infection in VTA. Inset: Co-labeled neurons indicated by closed arrowheads. A non-dopaminergic eYFP neuron is identified with an open arrowhead. (J) The percentage of eYFP-dopamine neurons does not differ between genotypes and in both genotypes there are significantly more eYFP-positive dopamine neurons in the VTA than in the substantia nigra (SNc) (Two-way mixed-design ANOVA, no interaction, F(1, 8) = 1.89, p = 0.20; no effect of genotype, F(1, 8) = 0.04644, p = 0.83; main effect of subregion, F(1, 8) = 23.84, p = 0.0012.) (K) Distribution map of stereological markers for TH-positive/eYFP-positive neurons in the VTA and SNc. (L) Model of adolescent dopamine axon growth. A subset of dopamine axons continue to grow from the NAcc into the mPFC during adolescence. DCC receptors within mesolimbic dopamine neurons promote target recognition events in the NAcc, preventing their ectopic growth into the mPFC.