Figure 4.
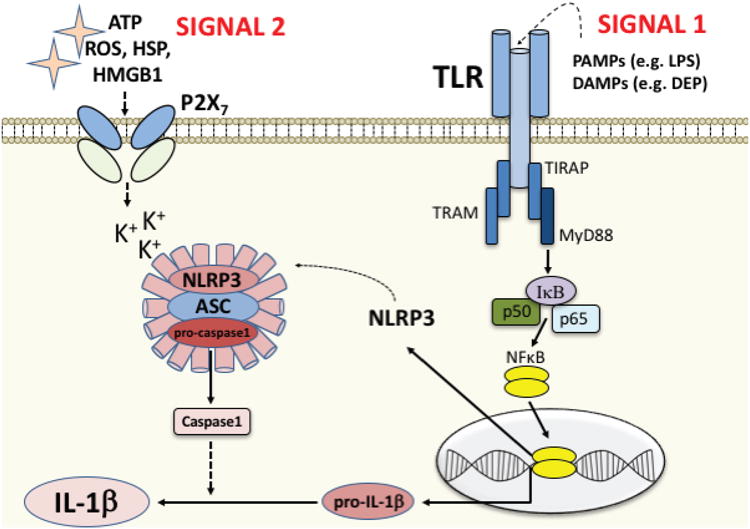
The process of cleaving pro-IL-1β into the mature, active protein often requires the formation of an inflammasome, an intracellular multiprotein complex that mediates processing and maturation of IL-1β via activation of caspase-1. This signaling cascade requires both a priming step (SIGNAL 1, acting on the TLR), and an activation step (SIGNAL 2, acting on, e.g. a purinergic receptor such as P2X7 or a variety of other scavenger receptors, see Hornung and Latz, 2010). SIGNAL 1 candidates include both exogenous ligands/PAMPs such as LPS, and endogenous “danger” signals/DAMPs that are released in response to toxins such as DEP (or cellular damage induced by DEP). Ligation of the TLR by SIGNAL 1 initiates the intracellular signaling cascade to activate the transcription factor NFkB and the subsequent production of pro-IL-1β, as well as Nod-Like-Receptor family Pyrin domain containing 3 (NLRP3). NLRP3 then translocates to the cytosol to form a complex with the proteins ASC and pro-caspase1, a critical step in the formation of the inflammasome. SIGNAL 2 candidates include ATP, reactive oxygen species (ROS), heat shock proteins (HSP), HMGB1, and many others, released in response to cellular distress or tissue damage. This second signal activates the inflammasome complex for cleavage of pro-IL-1β into IL-1β and release.
