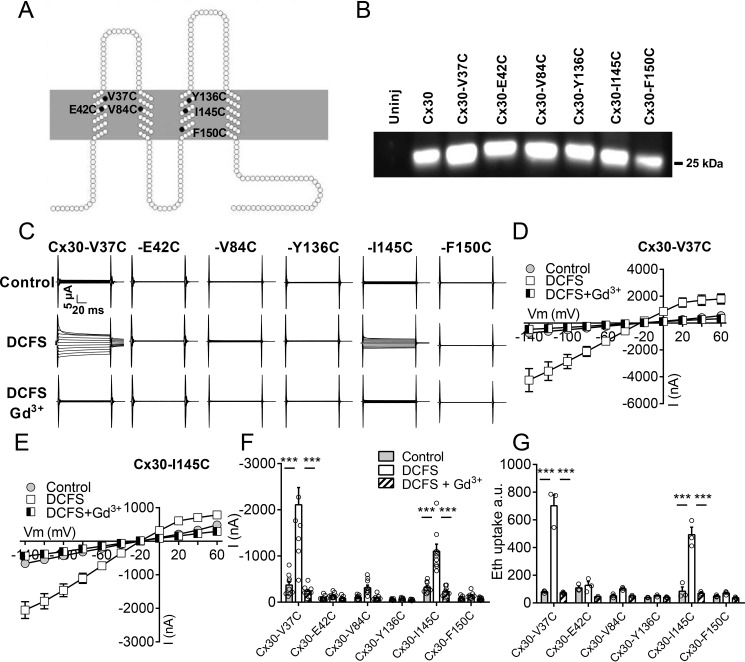
Figure 5.
Functionality of cysteine-mutated Cx30 hemichannels. A, diagram of the six residues in Cx30 that were individually mutated to cysteine (Cx30-V37C, Cx30-E42C, Cx30-V84C, Cx30-Y136C, Cx30-I145C, and Cx30-F150C). B, plasma membranes were purified from uninjected (uninj) oocytes, oocytes expressing Cx30, or one of the six mutated forms of Cx30, subjected to SDS gel electrophoresis, and immunoblotted with anti-Cx30 antibodies. C, membrane currents were recorded on oocytes expressing the mutated Cx30 hemichannels in control solution after 1-min exposure to DCFS and DCFS containing 50 μm Gd3+. Membrane currents were recorded by application of 100-ms voltage steps from +60 to −140 mV in steps of 20 mV from a holding potential of −50 mV. D and E, summarized I/V curves are illustrated for Cx30-V37C–expressing oocytes (D, n = 9) and Cx30-I145C (E, n = 9). F, histogram of currents obtained at −80 mV (n = 9). G, ethidium uptake recorded after 60-min exposure to control solution, DCFS, or DCFS containing 50 μm Gd3+ (n = 3). Data in I/V curves and in the bar graphs are presented as mean ± S.E. The individual data points are shown by overlaid scatterplots in bar graphs. Statistical significance was tested using repeated measures two-way ANOVA with Bonferroni post hoc test. ***, p < 0.001.