Figure 1.
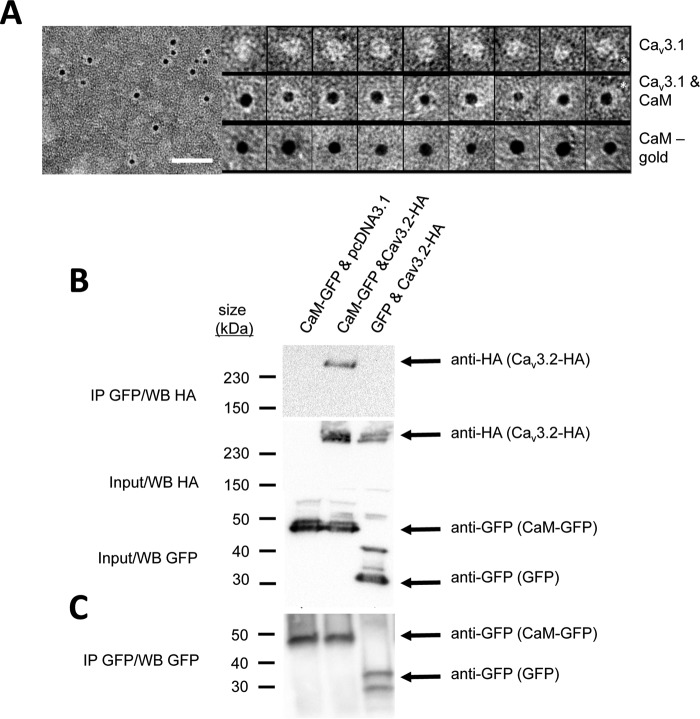
CaM associates with full-length Cav3 T-type channels illustrated by nano-particle cryoelectron microscopy (A) and co-immunoprecipitation (B). A, field of negatively stained (2% w/v uranyl acetate) full-length Cav3.1 in complex with CaM in the presence of 1.5 mm Ca2+. Scale bar is 50 nm. Left panel, CaM has a biotin label that binds streptavidin gold (5 nm). Protein appears white, and the gold that is electron-dense is black. Right panel, top row, montage of purified Cav3.1 particles (white density) presenting different views of the channel. Asterisk indicates “tail” domain that we have previously determined corresponds to the C terminus (38). Middle row, montage of Cav3.1 particles with CaM–biotin bond indicated by the presence of streptavidin gold (black sphere). Bottom row, control images of CaM–biotin–streptavidin gold particle without Cav3.1. Observed relative differences in Cav3.1 and streptavidin gold particle sizes relate to the orientation and contrast of each Cav3.1 channel particle adhered to the EM support film and the size variation in the streptavidin gold particles (3–6 nm with nominal size of 5 nm, according to the manufacturer). B, CaM–GFP bound to anti-GFP magnetic beads associates with hemagglutinin (HA)-tagged Cav3.2 channel (top panel, middle lane) as illustrated by HA antibody labeling (259-kDa band) of the Cav3.2 channel co-immunoprecipitant (IP) bound to beads. 259-kDa HA-tagged Cav3.2 channel band does not appear as a co-immunoprecipitant in the Western blot without co-expression of pCDNA3.1 plasmid inserts containing HA-tagged Cav3.2 channel (top panel, left lane) or without co-expression of CaM–GFP (top panel, right lane) in HEK-293T cells. Middle panel illustrates anti-HA antibody staining of the 259-kDa HA-tagged Cav3.2 channel of a replicate experiment of input proteins for the Western blot shown in the top panel without co-immunoprecipitation. Bottom panel illustrates anti-GFP antibody staining of the 44.2-kDa GFP-tagged CaM or GFP alone (27 kDa), in a replicate experiment of input proteins for the Western blot shown in the top panel without co-immunoprecipitation. C, CaM–GFP (left two lanes, 44.2 kDa) and GFP alone (right lane, 27 kDa) bound to anti-GFP magnetic beads as illustrated by anti-GFP antibody labeling (259-kDa band) of the Cav3.2 channel co-immunoprecipitant bound to beads. GFP alone generated two bands on the Western blot, which may result from differing post-translational modifications. Co-immunoprecipitation experiments were carried out in 33.3 μm CaCl2, pH 7.4. Vector for HEK-293T cell expressed inserts for Western blotting (Cav3.2–HA, EGFP, CaM–pGFP) were contained in pcDNA3.1. Membranes were stained with Ponceau red following protein transfer to evaluate the protein content in each lane. Co-immunoprecipitation experiments were carried out in 33.3 μm CaCl2, pH 7.4. Vector for HEK-293T cell expressed inserts for Western blotting (Cav3.2–HA, EGFP, CaM–pGFP) were contained in pcDNA3.1. Membranes were stained with Ponceau red following protein transfer to evaluate the protein content in each lane.