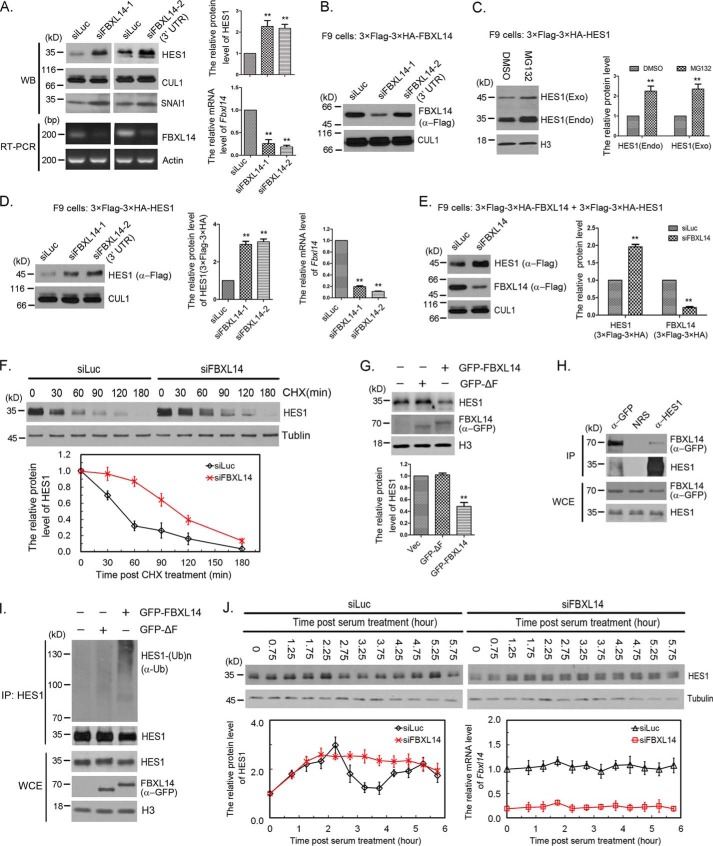
Figure 3.
FBXL14 was the F-box for SCF complex-mediated HES1 ubiquitination and degradation. A, FBXL14 regulated HES1 stability. The protein levels of HES1 and CUL1 were determined by Western blotting using indicated antibodies (left panel) and quantified (right panel). The quantification of protein levels of HES1 were measured as in Fig. 1A. FBXL14 mRNA inhibition was assessed by semi-quantitative RT-PCR. Right panel, the mRNA level of FBXL14 was examined using quantitative real-time PCR. The error bars represent S.D. from five independent experiments. The statistical difference between control group (Luc) and experimental groups (FBXL14) was measured by paired two-sided Student's t test (**, p < 0.01). B, the efficacy of FBXL14 siRNAs. Stably expressing 3× FLAG–3× HA–FBXL14 F9 cells were transfected with 50 nm FBXL14-1 or FBXL14-2 siRNAs as indicated for 45 h, and the cells were harvested for Western blotting. The protein levels of exogenous FBXL14 and CUL1 were determined by Western blotting as indicated. C, the protein level and stability of exogenous and endogenous HES1 in stably expressing 3× FLAG–3× HA–HES1 F9 cells. The protein levels of exogenous and endogenous HES1 were determined by Western blotting using anti-HES1 antibody (left panel) and quantified as in Fig. 1A, which was normalized to histone H3 (right panel). The statistical difference between control group (DMSO) and experimental groups (MG132) was measured as in A. D, FBXL14 regulated the stability of exogenous HES1. Stably expressing 3× FLAG–3× HA–HES1 F9 cells were transfected with luciferase or FBXL14 (FBXL14-1 and FBXL14-2) siRNAs for 45 h. The protein levels of exogenous HES1 (3× FLAG–3× HA) and CUL1 were determined by Western blotting using indicated antibodies (left panel) and quantified (middle panel). Right panel, the mRNA level of FBXL14 was examined and quantified as in A. The error bars represent S.D. from three independent experiments. The statistical difference between control group (Luc) and experimental groups (FBXL14) was measured as in A. E, deletion of FBXL14 stabilized exogenous HES1. Stably co-expressing 3× FLAG–3× HA–HES1 and 3× FLAG–3× HA–FBXL14 F9 cells were transfected with FBXL14 (FBXL14-1) or luciferase siRNAs for 45 h, and cell lysates were immunoblotted using anti-FLAG antibody. Right panel, the protein levels of exogenous HES1 (3× FLAG–3× HA) and FBXL14 (3× FLAG–3× HA) were quantified. The error bars represent S.D. from three independent experiments. The statistical difference between control group (Luc) and experimental groups (FBXL14) was measured as in A. F, silencing of FBXL14 resulted in stabilization of HES1. F9 cells were transfected with luciferase or FBXL14 siRNAs for 45 h, and the cells were treated with CHX (100 μg/ml) for the indicated times. The protein levels of HES1 and tubulin were determined by Western blotting. Bottom panel, the relative protein level of HES1 was quantified and normalized to tubulin. G, FBXL14 promoted HES1 degradation. F9 cells were transfected with pEGFP-C2 (Vec), pEGFPC2–FBXL14 ΔF, or pEGFPC2–FBXL14 for 48 h, and endogenous HES1 and exogenous FBXL14 were detected by Western blotting. Bottom panel, the protein levels of HES1 was quantified. The error bars represent S.D. from three independent experiments. The statistical difference between control group (Vec) and experimental groups (GFP-ΔF and GFP–FBXL14) was measured as in A. H, FBXL14 interacted with HES1. F9 cells were transfected with pEGFPC2–FBXL14 for 48 h, and the cells were treated with 20 μm MG132 for 3 h, harvested, and analyzed by co-immunoprecipitation (IP) and Western blotting. The experiment was biologically repeated at least three times. I, SCF complex promoted HES1 polyubiquitination in vivo. GFP–FBXL14, GFP–FBXL14 ΔF, and pEGFP-C2 were expressed in F9 cells for 44 h, and MG132 was added 3 h before cell harvesting to stabilize ubiquitylated protein. Lysates were immunoprecipitated with HES1 antibody under denaturing conditions (radioimmune precipitation assay buffer), and immunocomplexes were analyzed with the indicated antibodies. Immunoblots of whole-cell extracts were shown at the bottom. J, silencing of FBXL14 disrupted HES1 oscillation. F9 cells were transfected with luciferase or FBXL14 siRNAs for 24 h followed by serum-withdrawal starvation for 18.5 h. The cells were harvested at the indicated times once serum was supplemented. The protein level of HES1 was examined at the indicated times once serum was supplemented to the medium. Bottom panel, the quantification of relative protein level of HES1 and the relative mRNA level of Fbxl14. The experiment was biologically repeated at least five times.