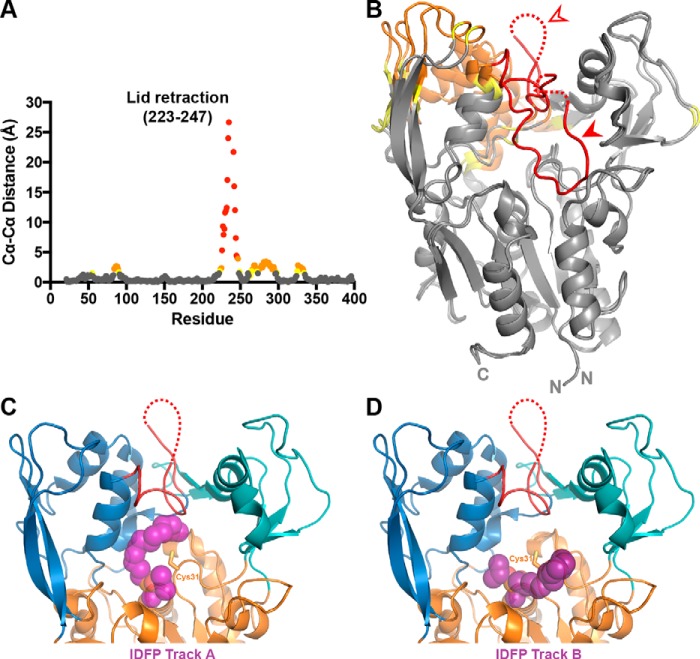
Figure 6.
Changes associated with lid-opening and IDFP binding. A, changes in Cα–Cα distance are graphed for residues between our closed LCAT structure and the open-2Fab structure, aligned on their α/β hydrolase domains. There is a gap in the plot due to unmodeled residues in the two compared crystal structures (residues 236–240). Residues with Cα–Cα differences above 4 Å are colored in red, distances between 2 and 4 Å in orange, and distances 1.5 and 2 Å in yellow. B, Cα–Cα distances from A mapped onto the structures with the same color scheme. The filled arrowhead points to the closed lid, and the open arrowhead points to the open lid. C and D, IDFP-bound LPLA2 (PDB code 4X91) was aligned with the open-2Fab structure using PyMOL. The two tracks observed for IDFP, as observed in the LPLA2 structure, are shown with track A in C and track B in D. IDFP is modeled as purple spheres bound to the active-site serine.