Figure 7.
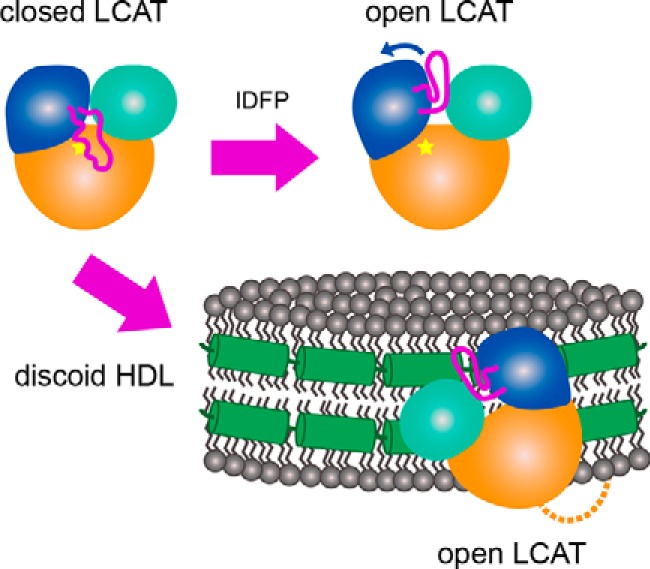
A model describing the proposed conformational change induced by IDFP and HDL. In solution, LCAT is in a closed state (top left) where the dynamic lid is extended over the active site (indicated by the star). As IDFP or other large hydrophobic molecules bind, we propose that the lid retracts into an open conformation (top right). The bottom arrow indicates HDL binding, which based on these data would involve the N-terminal region (dashed line), lid (magenta), and membrane-binding domain (teal). LCAT would then be positioned such that the hydrophobic active site is exposed to HDL to extract substrate for acyl transfer. The specific region of LCAT that contacts apoA-I (green helices) on HDL is unknown.
