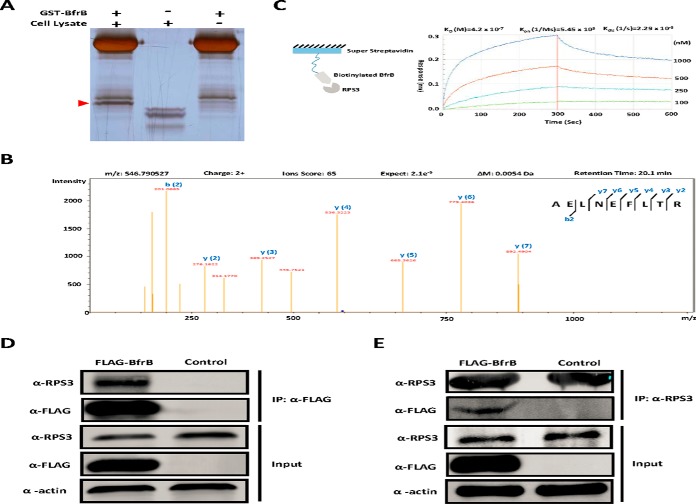
Fig. 2.
Validation of the interaction between BfrB and RPS3. A, GST Pull-down of BfrB. GST tagged BfrB was incubated with PMA- differentiated THP-1 cell lysates followed by GST Pull-down, whereas GST only was included as the negative control. The unique band was then trypsin digested and subjected to MS/MS analysis. B, RPS3 was identified as the major binder of BfrB by LC-MS/MS analysis. C, The binding kinetics of BfrB to RPS3 measured by BLI assay. Biotinylated BfrB was immobilized on a streptavidin-coated biosensor and exposed to RPS3 in buffer. The binding was measured by coincident changes in the interference pattern. Results presented are representative of three experiments. D, Co-immunoprecipitation of RPS3 with FLAG-BfrB. Samples were immunoprecipitated with α-FLAG antibody to capture BfrB and immunoblotted with α-RPS3 to detect RPS3. Experiments were carried out in triplicate. E, Co-immunoprecipitation of FLAG-BfrB with RPS3. Samples were immunoprecipitated with α-RPS3 antibody to capture RPS3 and immunoblotted with α-FLAG antibody to detect BfrB. Experiments were carried out in triplicate.