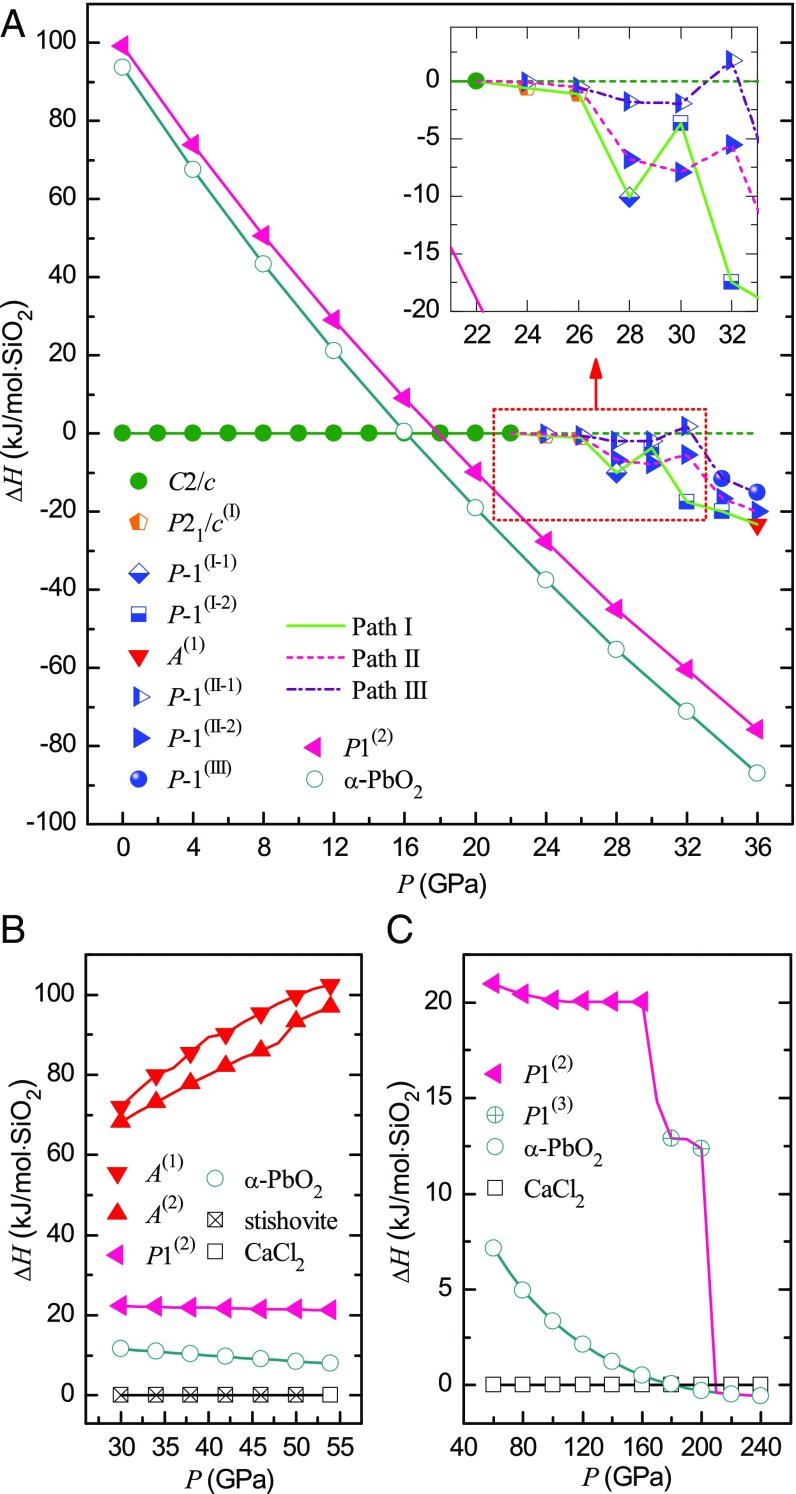
Fig. 2.
(A) Relative enthalpy ∆H of different phases along three phase-transition paths in the pressure range of 22–36 GPa. ∆H is the difference between the enthalpy of a phase and that of coesite at the same pressure. (B) Comparison of enthalpies of some amorphous and HPO phases in the pressure range of 30–54 GPa. For convenience, the enthalpies of stishovite and CaCl2 phase of silica are set to be zero at each pressure. (C) Relative enthalpy ∆H versus pressure from 60 to 240 GPa. ∆H is the difference between the enthalpy of a phase and that of CaCl2 phase at the same pressure along the compression course. Note: Coesite changes into P21/c(I) or P-1(II-1) phase at ∼22 GPa. So, the equation of state of coesite is extrapolated to higher pressure using the third-order Birch–Murnaghan equation (38), which is used as reference state in A.