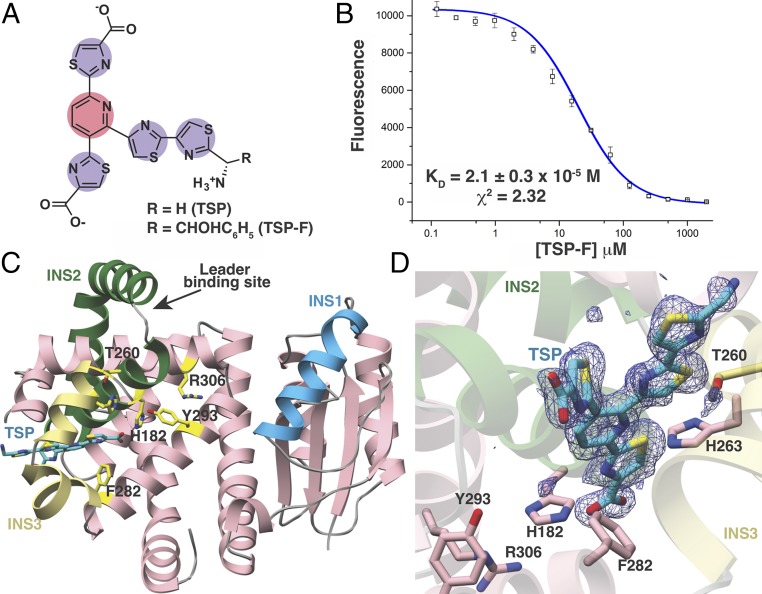
Fig. 3.
Structural and binding studies of the PbtD–TSP complex. (A) Chemical structure of the trisubstituted pyridine (TSP) core produced by acid hydrolysis of GE2270A. (B) Binding of TSP to PbtD as measured by the changes in FRET upon complex formation. (C) Overall structure of PbtD in complex with TSP shows that residues in insertion 3 (yellow) become ordered upon ligand binding. (D) Simulated annealing difference Fourier map (Fobs – Fcalc) contoured at 2.5σ showing the TSP core binds to a region near insertion 3 (yellow) in PbtD. Active-site residues implicated in catalysis, without influencing leader peptide binding, are shown in stick figures.