Fig. 2.
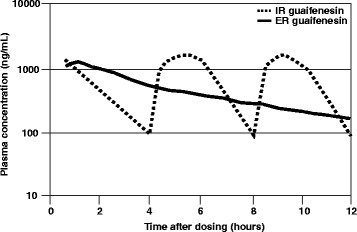
Schematic pharmacokinetic profile of extended-release (ER) vs immediate-release (IR) guaifenesin formulations. Extended-release (ER) guaifenesin (blue line) attained bioequivalent plasma concentrations to those obtained with 3 immediate-release (IR) guaifenesin doses (orange line). The unique bi-layer tablet formulation comprises an IR layer that permits immediate release of guaifenesin to rapidly attain maximum plasma concentrations (Cmax), and an ER layer that permits sustained release of guaifenesin to maintain prolonged blood plasma levels of guaifenesin over 12 h. Figure adapted from Vilson and Owen, 2013 [20]
