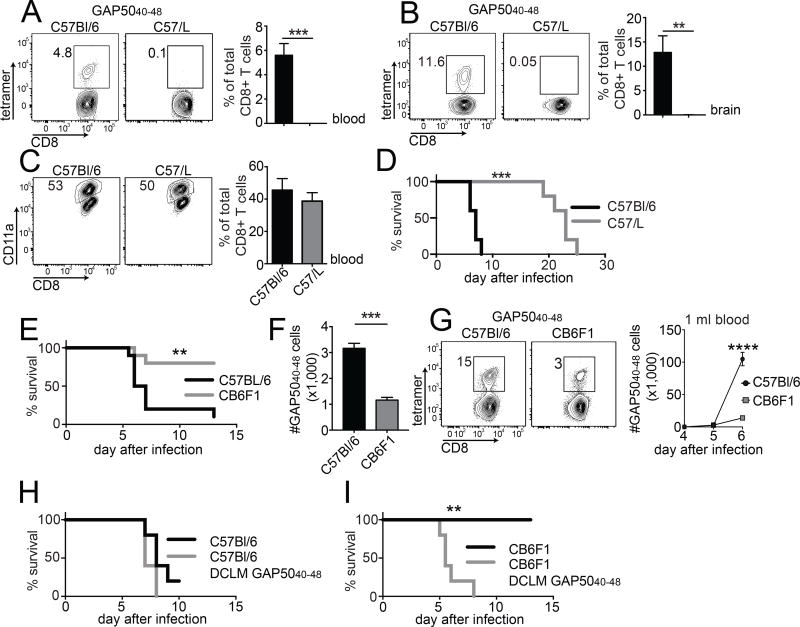
Figure 6. Vβ8.1 is required for the generation of GAP5040–48-specific CD8+ T cells and the development of ECM.
(A, B) C57Bl/6 and C57/L mice were infected with 1,000 P. berghei ANKA sporozoites. H-2Db-GAP5040–48 tetramer+ cells were quantified in the blood (A) and brains (B) at day 7 post-infection (representative plots: left; summary graph: right). (C) Magnitude of the activated CD8+ T cell response at day 7 post-infection, expressed as % CD11ahi CD8lo of total CD8+ T cells (representative plots: left; summary graph: right). Naive control values were subtracted from individual values. Data represent two independent experiments (n = 3 mice/group). Bars depict mean ± SD. Significance was assessed using an unpaired, two-tailed t test (**p < 0.01, ***p < 0.001). (D) Survival curves for C57Bl/6 mice and C57/L mice after infection with P. berghei ANKA. Data represent three independent experiments (n = 5 mice/group). Significance was assessed using the Mantel-Cox log rank test (***p = 0.001). (E) C57Bl/6 and CB6F1 mice were infected with 106 P. berghei-parasitized red blood cells (pRBCs). Mice were monitored for the development of ECM symptoms and scored for survival over a period of two weeks. Data represent three independent experiments (n = 5 mice/group). Significance was assessed using the Mantel-Cox log rank test (**p = 0.0025). (F) GAP5040–48-specific CD8+ T cells were tetramer-enriched from naive C57Bl/6 and CB6F1 mice and quantified to estimate repertoire size. Cumulative results are shown from two independent experiments (total n = 4 mice/group). Bars depict mean ± SEM. Significance was assessed using an unpaired, two-tailed t test (****p <0.0001). (G) C57Bl/6 and CB6F1 mice were infected with 106 pRBCs. The GAP5040–48-specific CD8+ T cell response was followed in the blood of infected mice using H-2Db-GAP5040–48 tetramers. All C57Bl/6 mice succumbed to ECM by days 6 and 7, while all CB6F1 mice survived. Data represent two independent experiments (n = 5 mice/group). Bars depict mean ± SD. Significance was assessed using an unpaired, two-tailed t test (****p < 0.0001). (H, I) C57Bl/6 and CB6F1 mice were injected IV with 5 × 105 GAP5040–48 peptide-coated DCs and then infected 7 days later with recombinant attenuated LM-GAP5040–48. At a memory time point after LM infection, DC-LM-GAP5040–48-immunized or non-immunized mice were infected with 106 pRBCs. Survival curves are shown for C57Bl/6 mice (H) and CB6F1 mice (I). Data represent two independent experiments (n = 5 mice/group). Significance was assessed using the Mantel-Cox log rank test (p = 0.0993 in panel D; **p = 0.0018 in panel E).