Abstract
Particulate vaccines are emerging promising technologies for the creation of tunable prophylactics against a wide variety of conditions. Vesicular and solid biodegradable polymer platforms, exemplified by liposomes and polyesters, respectively, are two of the most ubiquitous platforms in vaccine delivery studies. Here we directly compared the efficacy of each in a long-term immunization study and in protection against a model bacterial antigen. Immunization with poly(lactide-co-glycolide) (PLGA) nanoparticles elicited prolonged antibody titers compared to liposomes and alum. The magnitude of the cellular immune response was also highest in mice vaccinated with PLGA, which also showed a higher frequency of effector-like memory T cell phenotype, leading to an effective clearance of intracellular bacteria. The difference in performance of these two common particulate platforms is shown not to be due to material differences but appears to be connected to the kinetics of antigen delivery. Thus, this study highlights the importance of sustained antigen release mediated by particulate platforms and its role in the long-term appearance of effector memory cellular response.
Keywords: Liposome, PLGA, Nanoparticle, Persistence, Vaccine, Memory T cells
1. Introduction
Synthetic particulate vaccine delivery systems are promising modalities for shaping immune responses against a number of disease states [1–10]. There are several significant advantages to pursuing this alternative methodology for vaccine development. First, the size of these systems can be controlled down to the nanometer scale, enabling transport through extracellular and intracellular biological barriers. Second, antigens can be encapsulated and protected for delivery by various routes of administration, such as oral or intranasal [11–13]. Third, control over the particle surface chemistry can enable modular functionalities to be easily introduced, such as attachment of shielding polymers [14–16] or varying the density of targeting ligands to receptors on professional antigen-presenting cells [17]. Fourth, the capability of tuning release rates of antigen by varying the chemistry or preparation procedure of such systems may be an attractive alternative to the booster requirements in vaccination regimens. Finally, particulate vaccines may be prepared to facilitate endosomal disruption after internalization; enabling cross presentation of antigen for eliciting both CD4+ and CD8+ T cell responses; an important consideration for induction of a comprehensive antigen-specific immune response [18].
Particulate vaccines may be constructed in a variety of ways using different materials [19], but the majority of these systems fall into two general classes: solid biodegradable systems or vesicular systems. As such, liposomes and polyester particles, exemplified by poly(lactic-co-glycolic acid) (PLGA), have been extensively investigated in vaccine delivery applications. Surprisingly, given the long research history with both systems, very few studies have directly compared their immunological response or vaccination efficacy [20–22], especially with respect to the memory phenotype of the immune response that develops after administration.
In this study, we directly compared long-term vaccination efficacy between liposomes and PLGA nanoparticles encapsulating the model antigen ovalbumin, (OVA). Liposomes are spherical vesicles composed of a phospholipid bilayer surrounding an aqueous core; first proposed as vaccine adjuvants over 35 years ago by Gregoriadis [23]. The soluble antigen protein is incorporated in the hydrophilic core of the particle and released after disruption of the lipid bilayer. Solid polymer particles composed of the polyester PLGA have been extensively studied in vaccine applications for over 20 years [24,25]. Here the antigen is entrapped in the polymer core and is released upon polymer degradation. Given the major differences in antigen incorporation and release, we sought to determine the long-term vaccine immune response affected after administration of these two representative, classes of particles. To address this question, we monitored humoral responses over the course of several months and compared cellular responses from vaccinated mice to ex vivo antigen challenge. We then tested immunological memory using recall experiments with an OVA-expressing intracellular bacteria, Listeria monocytogenes.
2. Materials and methods
2.1. Materials
Liposome components (cholesterol and Lα phosphatidylcholine-PC) were purchased from Avanti Polar Lipids (Alabaster, AL). PLGA (50:50) was bought research grade from Durect (Pelham, AL) at two viscosities: 0.10 and 1.15 dL/g. Ovalbumin (OVA) (Grade V), bovine serum albumin (BSA), chloroform, and poly(-vinyl alcohol) were purchased from Sigma (St. Louis, MO). Methanol, NaOH, Triton-X, phosphate buffered saline (PBS), and methylene chloride were obtained from Omnisolv (Salisbury, NC), JT Baker (Phillipsburg, NJ), American Bioanalytical (Natick, MA), Gibco (Carlsbad, CA), and Fisher Scientific (Pittsburgh, PA), respectively.
OVA-expressing L. monocytogenes (LM-OVA) was a generous gift from Hao Shen (University of Pennsylvania). A protocol for the generation of LM-OVA can be found elsewhere [26].
2.2. Liposome fabrication and characterization
Liposomes were prepared with PC and cholesterol at a 2:1 M ratio by the extrusion method. PC and cholesterol were mixed and solvents (chloroform and methanol) were evaporated in the presence of nitrogen gas. The resulting layer was hydrated with 5 mg/ml OVA in PBS. Blank liposomes were made by hydration with 1 × PBS followed by vortexing and agitation on an orbital shaker for 1.5 h. Extrusion was performed by 5 successive passes first through a 1 um filter, then 500 nm filter, and finally through a 200 nm filter. The resultant unilamellar liposomes were collected by ultracentrifugation at 100,000 × g for 1h and resuspended in PBS then frozen at −20 °C until needed.
To assess protein content in liposomes, a weighed aliquot of lyophilized product was denatured in 1 ml of 0.2N NaOH + 10% Triton-X for 1 h. Protein content was measured by the Micro BCA assay (Pierce). Blank liposomes without encapsulated protein and soluble OVA were used to create a standard curve. Release from liposomes was measured by rotating aliquots of liposomes in PBS over 6 weeks at 37 °C. Each week, aliquots were removed and centrifuged; and the supernatant was stored at −20 °C. At the end of 10 weeks, samples were thawed and OVA was quantified by the Micro BCA assay.
2.3. PLGA nanoparticle fabrication and characterization
Nanoparticles were prepared by a double emulsion method as previously described [27]. In the first emulsion, a highly concentrated solution of OVA at 100 mg/mL was added dropwise to 100 mg of PLGA in methylene chloride while vortexing. The first emulsion was then added dropwise to a 2.5% PVA solution in water. Both emulsions were sonicated on ice for 30 s using a Tekmar Sonic Distributor fitted with a CV26 sonicator (38% amplitude) on ice. Nanoparticles were allowed to harden while stirring vigorously in 100 mL of 0.3% PVA for 3 h at room temperature. Particles were washed three times with deionized water by centrifugation at 18,500 × g, followed by freezing and lyophilization. Nanoparticles were stored at −20 °C until further use.
Protein content was assayed by dissolving PLGA nanoparticles in 0.2N NaOH overnight. Similar to the liposome procedure, protein concentrations was quantified by the Micro BCA Assay using unloaded nanoparticles and free soluble OVA as a standard. OVA release from PLGA nanoparticles was performed by incubating nanoparticles in PBS at 37 °C and sampling supernatant weekly. Supernatant was stored frozen at −20 °C, until protein content was detected by the Micro BCA Assay after 10 weeks.
2.4. Animal immunization
Mice used in this study were housed in pathogen-free facilities maintained by the Yale Animal Resource Center staff. Female C57Bl/6 mice at 6–8 weeks of age were immunized subcutaneously at the base of the tail with 100 μg of OVA encapsulated either in liposomes or PLGA nanoparticles. Mice received a single immunization with no booster dose. Mice were retro-orbitally bled bi-weekly for serum samples and euthanized after 11 weeks.
2.5. Antibody analysis
Blood samples were incubated overnight at 4 °C then centrifuged at 1000 × g. Serum was isolated and stored at −80 °C. Samples from all time points were analyzed simultaneously by ELISA. Briefly, 5 μg OVA in PBS was added to wells of 96-well high protein binding plates and incubated overnight at 4 °C. Next, plates were blocked with 5% BSA in PBS for 1 h at RT. Serum samples were incubated at various dilutions in blocking buffer for 2 h at RT. Next, anti-OVA-IgG/IgG1/IgG2b-HRP detection antibodies (Jackson Immunoresearch) were added for 1 h at RT. Finally, plates were developed with TMB substrate. The reaction was stopped with 1N HCl and absorbance was quantified at 450 nm. Titer was calculated as the inverse dilution at which the sample matched that of the average absorbance (plus 2 standard deviations) from control samples (mice receiving particles alone). Values were transformed into log scale to derive a linear curve of dilution versus absorbance.
2.6. Analysis of the IFN-γ response in splenocytes
Splenocytes single cell suspensions were prepared by forcing the excised spleen through a filter. Next, red blood cells were lysed by treatment with an ammonium chloride solution followed by centrifugation and washing with 1 × PBS. Cells were stimulated with OVA for 72 h in “complete media” - RPMI with 10% fetal calf serum (FCS), 1% glutamine, 0.1% β-mercaptoethanol, and 2% penicillin/streptomycin. Supernatant was collected and analyzed for IFN-γ secretion by ELISA (BD Pharmingen).
2.7. Antigen-specific lymphocyte activity after a bacterial challenge
Mice were immunized as above with OVA-encapsulating liposomes or PLGA nanoparticles or OVA adsorbed to the commercially available aluminum hydroxide gel, Alhydrogel (Accurate Chemical). At 90 days post-immunization, mice were transferred to a biosafety level 2 + facility where OVA-expressing L. monocytogenes (LM-OVA) was administered intravenously by tail injection. Mice were divided into two groups, with Group I receiving a higher dose of 100,000 CFU (optimal for assaying of bacterial titers after 3 days) and another receiving a non-lethal dose of 50,000 CFU (Group II) for intracellular cytokine and biomarker analysis.
Group I spleens were collected 3 days post-challenge. Spleens were first weighed, and single cell suspensions were prepared, without red blood cell lysis. Splenocytes were kept in complete media without antibiotic supplement and the suspension was mixed with a 1:1 ratio of 1% Triton-X 100 in sterile deionized water for 3 min. After cell permeabilization, the suspension was diluted in media and plated onto BBL Brain Heart Infusion agar (BD). After an overnight incubation at 37 °C, bacterial colonies were enumerated to calculate CFU.
At 7 days post-challenge, splenocytes from Group II mice were stained for surface markers for 30 min on ice, then fixed (Cytofix – BD Biosciences) and analyzed by flow cytometry (LSRII – BD Biosciences). Biomarkers were selected for analysis of T cell helper or cytotoxic phenotype (CD4, CD8), activation (CD44, CD62L) and memory phenotype (KLRG1, CD27, CD127) (all eBioscience), and a tetramer for T cells specific for the dominant OVA CD8+ peptide, SIINFEKL (Beckman Coulter). In parallel, splenocytes from each sample were analyzed for intracellular cytokine expression. For intracellular cytokine analysis, cells were incubated with Bredfeldin A (eBioscience) (1:1000) and SIINFEKL (GenScript) (400 ng/ml) in complete media for 8 h at 37 °C to induce antigen-specific cytokine production. Next, cells were stained for extracellular markers CD4 and CD8 (eBioscience), fixed, permeabilized (PermWash, BD), and intracellularly stained for IFN gamma (eBioscience), followed by flow cytometry analysis.
3. Results
3.1. Nanoparticle characterization
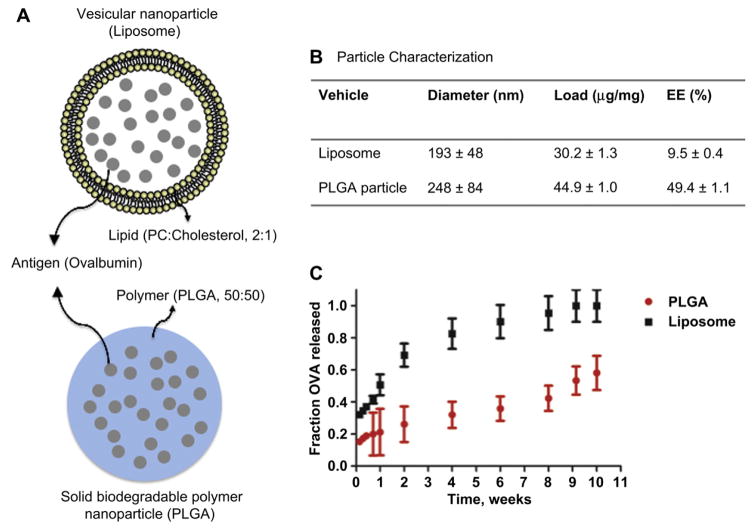
A schematic of the two delivery platforms used in this study is depicted in Fig. 1A. Common to both platforms is that the protein antigen resides within the interior of the nanoparticles, protected from the outside microenvironment: within the polymer matrix in PLGA nanoparticles and the aqueous core in liposomes. In this work, the formulation conditions were adjusted to yield similar size liposomes and PLGA nanoparticles and similar protein loadings (Fig. 1B). PLGA nanoparticles had a slightly higher protein load and greater polydispersity compared to the liposomes, which on average were slightly smaller than PLGA nanoparticles but were more monodisperse due to the extrusion fabrication method (Fig. 1B). The protein encapsulation efficiency of these liposomes was markedly lower than that of the PLGA nanoparticles. The release profiles of the two particulates are shown in Fig. 1C. Liposomes released their contents at a significantly faster rate than the PLGA nanoparticles.
Fig. 1.
Nanoparticle characterization. (A) Schematic of liposomes and PLGA nanoparticles highlight the general structural differences between the vehicles. Liposomes entrap soluble protein antigen (OVA) in an aqueous core surrounded by a phospholipid and cholesterol bilayer. PLGA nanoparticles encapsulate OVA in a biodegradable solid polymeric matrix. (B) Particle Characterization. Particles were formulated to have relatively similar size and loading (mass of OVA/mass of particles). PLGA particles have much higher encapsulation efficiency (EE) [(experimental load/theoretical maximum load) × 100] compared to liposomes. (C) OVA release profiles from liposomes and PLGA nanoparticles. Curves represent release from nanoparticles at 37 °C in 1 × PBS. Release was performed in triplicate over 10 weeks.
3.2. Persistence of humoral and cellular immune responses after vaccination with PLGA nanoparticles in comparison to liposomes
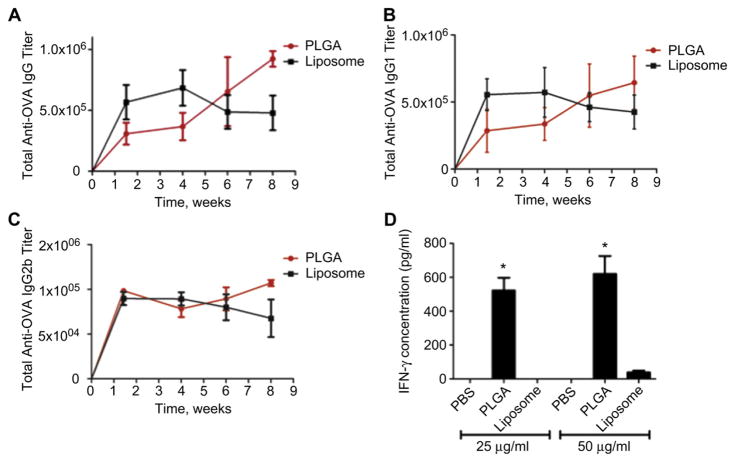
Mice were immunized subcutaneously with the same amount of antigen encapsulated in either liposomes or PLGA nanoparticles and evaluated for subsequent immune responses (Fig. 2). Mice immunized with liposomes displayed an early peak in circulating OVA-specific IgG around 4 weeks, followed by a regression in titers (Fig. 2A). Alternatively, mice administered PLGA nanoparticles showed higher antibody responses at 6 weeks and beyond. Further analysis indicated that this difference in antibody levels was due primarily to IgG1 (Fig. 2B), as IgG1 levels displayed the same trend and magnitude as total IgG. IgG2b curves (Fig. 2C) were similar between the groups, showing much lower titers than IgG1, as to be expected from this isotype.
Fig. 2.
Humoral and cellular responses after vaccination with liposomes and PLGA nanoparticles. Mice (N = 4) were subcutaneously administered a single dose of 100 μg of OVA encapsulated in liposomes or PLGA nanoparticles. Mice were bled approximately every 2 weeks and analyzed for antigen-specific (A) total IgG, (B) IgG1, and (C) IgG2b titers by ELISA. At 11 weeks, splenocytes were pulsed ex vivo with OVA at 25 and 50 μg/mL and analyzed for (D) IFN-γ in the supernatant after 72 h by ELISA. Data shown are from a single experiment that was performed twice with the same results. * Indicate P value less than 0.05.
At 11 weeks, T cell activity was assessed by pulsing splenocytes with the OVA antigen ex vivo and measuring the level of IFN-γ production by ELISA. As shown in Fig. 2D, IFN-γ responses from PLGA particle-vaccinated mice were higher than the liposome group. This is consistent with previous work, which showed enhanced splenocyte proliferation after a single dose of PLGA microspheres compared to multiple dose administration with similar liposomes at 6 and 14 weeks post-immunization [21].
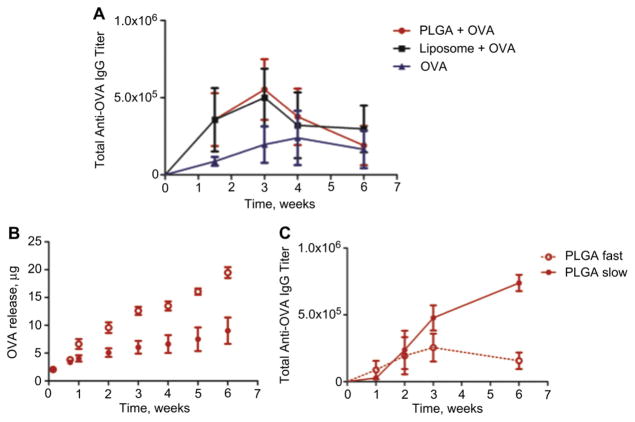
To ascertain if the differential in immune responses between the systems was due to an adjuvant effect that maybe associated with the material alone, we administered empty carriers with free protein. Interestingly, we observed nearly identical IgG production profiles from both the liposome and PLGA nanoparticle adjuvant systems (Fig. 3A). Both materials exhibited an early peak in IgG that may correlate with a larger supply of protein available to antigen-presenting cells at the time of injection compared to when encapsulated. This was followed by an abrupt decrease in titers, which may be due to the fact that the protein is cleared from the body or degraded when not encapsulated. Encapsulated OVA in liposomes resulted in a very similar profile to the unloaded carriers with soluble protein (Fig. 2A). This may be explained by the instability or fast release of protein from liposomes (Fig. 1B). In short, an initial burst release of protein or higher protein availability facilitated an initial higher titer level that decreased without continuous antigen availability. No ex vivo splenocyte activity was detected in any of these groups.
Fig. 3.
Comparative humoral responses after vaccination with liposomal and PLGA nanoparticles. Mice (N = 4) were subcutaneously vaccinated with 100 μg soluble OVA with and without unloaded PLGA nanoparticles or liposomes, and monitored for their circulating (A) total anti-OVA IgG over time in order to address adjuvant effects from the material. PLGA nanoparticles were fabricated using low inherent viscosity polymer that released contents at a faster rate (PLGA “fast”) and high viscosity polymer that released contents at a slower rate (PLGA “slow”). (B) The release profiles of particles in triplicate at 37 °C in PBS are shown over 6 weeks. Mice were vaccinated (N = 5) with a single s.c. injection and (C) circulating total anti-OVA IgG was measured in the serum by ELISA at various time points.
To address the role of release kinetics in the humoral immunological response, PLGA nanoparticles were made using polymer of two different inherent viscosities: 0.15–0.25 dL/g and 0.95–1.2 dL/g which released OVA at “fast” and “slow” rates, respectively (Fig. 3B). The “slow” polymer was the polymer used for all the other studies with PLGA throughout this report. Mice were vaccinated with these nanoparticles as above. Indeed the particles that released their load at a slower rate also presented the higher titers of circulating IgG over time (Fig. 3C).
3.3. Enhanced immunological memory recall from PLGA nanoparticles compared to liposomal formulation using a bacterial agent
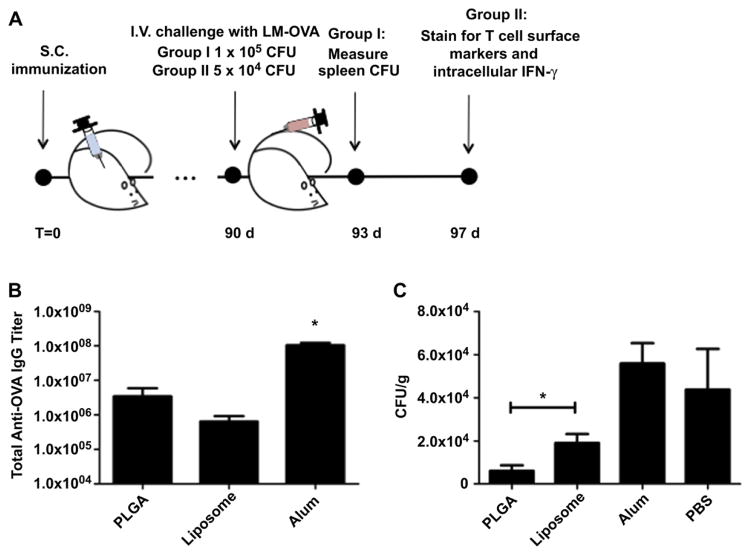
To better understand the T cell memory response and its effect on protection from subsequent infection after immunization, mice were challenged with OVA-expressing L. monocytogenes (LM-OVA), as described in Fig. 4A. At 13 weeks post-immunization with OVA adsorbed to alum, or encapsulated in liposome or PLGA nanoparticles, mice were analyzed for OVA-specific total IgG and intravenously injected with LM-OVA. At this time point, IgG titers from mice vaccinated with alum were more than an order of magnitude higher than those from vaccination with either type of delivery vehicle, while titers with PLGA continued to be higher than with liposomes (Fig. 4B).
Fig. 4.
Post-vaccination recall of Listeria monocytogenes (LM-OVA). (A) Study timeline. Mice received a single subcutaneous administration of OVA adsorbed to aluminum hydroxide or encapsulated in liposomes or PLGA nanoparticles. At 13 weeks, mice were divided into two groups(N = 3) and received intravenous (I.V) tail vein injections with either 1 × 105 (group I) or 5 × 104 (group II) CFU of LM-OVA. At 3 days post-challenge, splenocytes from Group I mice were assayed for bacterial titers. At 7 days post-challenge, splenocytes from group II mice were stained for effector T cell surface markers and intracellular IFN-γ and analyzed by flow cytometry. (B) Humoral response at time of challenge. Serum anti-OVA IgG was measured by ELISA. * Indicate a P value less than 0.001. C) LM-OVA titers in the spleen. Three days post-LM-OVA challenge, splenocytes from Group I mice were permeabilized and plated on agar overnight. Colony forming units (CFU) were calculated and normalized by mass of the organ. Study was repeated twice with same results. *Indicates a P value less than 0.05.
Bacterial titers in the spleen were determined in a cohort of mice 3 days post-recall. Immunization with PLGA nanoparticles elicited the highest bacterial clearance compared to liposomes and alum (Fig. 4C). Liposome immunization was more effective in bacterial clearance compared to alum (Fig. 4C). In fact, vaccination with OVA-adsorbed alum was as insufficient as no treatment in clearing the infection (Fig. 4C), despite high IgG titers in the serum (Fig. 4B). The reason for this is that LM-OVA infection is properly overcome by a cellular cytotoxic immune response (CD8+ T cell response) and not neutralizing antibodies. Indeed, alum is known for its tendency to promote a robust antibody and TH2-type biased response [28].
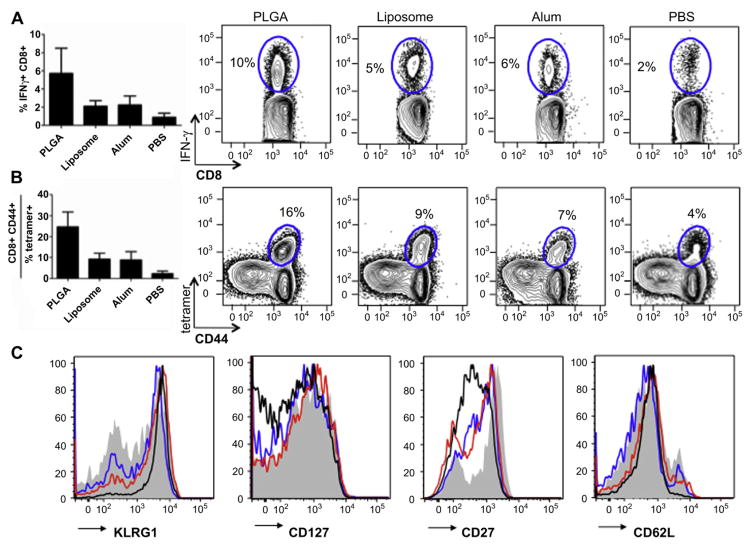
To evaluate the antigen-specific memory T cell responses in mice vaccinated with the various formulations, we analyzed splenocytes ex vivo 7 days post-recall. After pulsing with a dominant CD8+ OVA peptide, SIINFEKL, cells were stained intracellularly for IFN-γ. CD8+, IFN-γ+ populations were highest in mice vaccinated with PLGA compared to liposomes and alum, which were similar to each other (Fig. 5A). Regardless of the group, cells receiving no peptide did not produce any IFN-γ (data not shown). Additionally, T cells were stained for surface markers indicative of effector phenotypes and an MHC I SIINFEKL tetramer for OVA-specific T cell receptors. Again, immunization with PLGA correlated to the highest number of activated CD8+, CD44+, tetramer-specific T cells (Fig. 5B), with the overall response trend mirroring that of IFN-γ-expressing cells in Fig. 4A. Lastly, we analyzed antigen-specific CD8+ T cells from vaccinated mice for effector memory surface markers (Fig. 5C). T cells from mice administered PLGA nanoparticles appear the most effector-like in phenotype, with up-regulated KLRG1 and down-regulated CD62L, CD127 and CD27 (Fig. 5C).
Fig. 5.
Effector-like T cell analysis. Splenocytes from group II mice (N = 3) were pulsed with SIINFEKL and (A) IFN-γ expressing CD8+ T cell population was enumerated. (B) Activated antigen-specific CD8+ CD44 + T cells were also determined using a SIINFEKL tetramer. This population in C) was analyzed for surface markers indicative of an effector-like phenotype (KLRG1hi, CD127lo, CD27lo, CD62Llo). Shaded = PBS, Black = PLGA, Red = Liposome, Blue = Alum. Study was repeated twice with same results. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Nanoparticles composed of synthetic or natural building blocks are an emerging new modality for the creation of tunable, more effective vaccines [29]. Given that the most ubiquitous delivery vehicles are solid biodegradable polymer forms, exemplified by PLGA, and vesicular forms, typified by liposomes, we directly compared the simplest forms of these vehicles in vaccination and long-term protection using a model antigen system. Vaccination with OVA encapsulated PLGA nanoparticles led to a later peak in antigen specific antibodies that persisted for at least 13 weeks at higher levels than from immunization with liposomes encapsulating the same antigen. At the conclusion of the studies, antigen-specific lymphocytes from mice administered PLGA nanoparticles exhibited much higher ex vivo activity than liposomes. Immunological recall studies with an OVA-expressing intracellular bacteria demonstrated that vaccination with PLGA nanoparticles was more effective than liposomes at generating effector-like CD8+ T cells and as a result, led to rapid clonal expansion and subsequent clearance of a bacterial infection.
Additional studies were performed to address if these results were due to material or antigen release rate differences between the vehicles. Soluble OVA administered with blank liposomes or PLGA nanoparticles resulted in virtually identical serum IgG titers over time, suggesting that neither material is a better adjuvant over the other. Interestingly, the curves were reminiscent of that after vaccination with OVA-encapsulated in liposomes. This effect may be indicative of liposome instability in vivo. Considering the fast release of antigen from the liposomes, vaccination with OVA-loaded liposomes may lead to a large amount of soluble antigen being present shortly after the time of injection. Further studies held the material “constant”, by using PLGA nanoparticles that released OVA at different rates. As suspected, the PLGA nanoparticles that released at a slower rate had more robust humoral response.
The paramount goal of vaccination is to generate long-lived immunological memory that protects hosts from various infections [30–32]. Immunological memory consists of mainly two components, humoral (long-lived plasma cells and memory B cells) and cellular (memory CD4+ and CD8+ T cells) immunity [30–32]. Although viral and intracellular bacterial infections are best combated by cellular immune responses, current vaccines largely focus on generating neutralizing antibody responses [33,34]. Particulate vaccines provide a platform to deliver antigen as well as trigger both arms of immunological memory. In this study, we have shown that OVA-encapsulating PLGA nanoparticles provide better protective immunity to mice, highlighted by enhanced CD8+ T cell recall responses, than two other forms of vaccination regimens. This heightened secondary expansion usually reflects the increased quantity and/or quality of antigen-specific memory CD8+ T cells from initial immunization [34]. These effects of PLGA could be ascribed to the following scenarios. First, PLGA-mediated prolonged antigen release could offer prime-boost effect on memory CD8+ T cell generation [35,36]. It has been shown that antigen persistence during infection correlates with a stronger T cell response [37]. The slow release of protein from PLGA particles may more closely approximate the presence of soluble antigen during an infection. Second, PLGA may provide better adjuvant effects by eliciting proper cytokine milieus that favor higher number or quality of memory CD8+ T cell formation [10,30,38–41]. Lastly, PLGA generates memory CD8+ T cells that elicit immediate effector functions in response to pathogen entry and rapid secondary expansion to rapidly clear the infection [42,43]. In agreement with the later point, we found that secondary effector CD8+ T cell responses followed PLGA immunization displayed potent effector phenotypes and functionality characterized by higher KLRG1 expression and IFN-γ production.
Conventional liposomes have been previously shown to have weak cell-mediated responses [44], potentially due to instability issues in vivo. Others have mediated this effect with liposomal formulations modified in various ways. For example, different groups have taken measures to stabilize lipid-based systems by crosslinking and/or polymerization [45,46] or with agents such as alginate-poly(L-lysine) [47] and oil [48]. Interestingly, the stabilized preparations were more effective at generating antigen-specific cytotoxic T lymphocyte [48] and humoral [45] responses. Similarly, acid-sensitive preparations of liposomes were shown to enhance MHC Class I presentation [49] and subsequent CD8+ T cell activation.
5. Conclusions
The results of this work highlight new insights regarding the manner in which antigen is delivered for vaccination and its effect on long-term CD8+ T cell memory responses and efficacy in pathogen recall. Specifically, nanoparticle-mediated sustained antigen delivery is critical for the long-term quality and magnitude of the vaccine response. Targeting to antigen-presenting cells may further amplify this effect and/or compensate for vehicular instability. However, at the fundamental level, this study stresses the idea that slow, sustained antigen availability mediated by the choice of antigen vehicle may indeed be a sufficient factor in facilitating a long-term memory T cell response and recovery from pathogen infections.
Acknowledgments
We would like to gratefully acknowledge Joachim Hero, MPH; Jonathan Chen, Ph.D., Michael Look, Michael McHugh, Fiona Sharp and Alyssa Siefert for their assistance. This work was supported by NSF NIRT grant #CTS-0609326 to T.M.F.
Abbreviations
- PLGA
poly (lactic co-glycolic acid)
- TLR
Toll-like receptor
- OVA
Ovalbumin
- LM-OVA
Listeria monocytogenes-OVA construct
References
- 1.Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60:915–28. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Look M, Bandyopadhyay A, Blum JS, Fahmy TM. Application of nanotechnologies for improved immune response against infectious diseases in the developing world. Adv Drug Deliv Rev. 2010;62:378–93. doi: 10.1016/j.addr.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moon JJ, Suh H, Polhemus ME, Ockenhouse CF, Yadava A, Irvine DJ. Antigen-Displaying lipid-Enveloped PLGA nanoparticles as delivery agents for a Plasmodium vivax Malaria vaccine. PLoS One. 2012;7:e31472. doi: 10.1371/journal.pone.0031472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, Mark Saltzman W, et al. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27:3013–21. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell TJ, Palath N, DeRome ME, Tang J, Jacobs A, Boyd JG. Synthetic nanoparticle vaccines produced by layer-by-layer assembly of artificial biofilms induce potent protective T-cell and antibody responses in vivo. Vaccine. 2011;29:558–69. doi: 10.1016/j.vaccine.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Krishnamachari Y, Geary SM, Lemke CD, Salem AK. Nanoparticle delivery systems in cancer vaccines. Pharm Res. 2011;28:215–36. doi: 10.1007/s11095-010-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adair BM. Nanoparticle vaccines against respiratory viruses. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:405–14. doi: 10.1002/wnan.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–64. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 9.Petersen LK, Ramer-Tait AE, Broderick SR, Kong CS, Ulery BD, Rajan K, et al. Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants. Biomaterials. 2011;32:6815–22. doi: 10.1016/j.biomaterials.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Tongchusak S, Mizukami Y, Kang YJ, Ioji T, Touma M, et al. Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials. 2011;32:3666–78. doi: 10.1016/j.biomaterials.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 11.Ali J, Ali M, Baboota S, Sahani JK, Ramassamy C, Dao L, et al. Potential of nanoparticulate drug delivery systems by intranasal administration. Curr Pharm Des. 2010;16:1644–53. doi: 10.2174/138161210791164108. [DOI] [PubMed] [Google Scholar]
- 12.Chadwick S, Kriegel C, Amiji M. Nanotechnology solutions for mucosal immunization. Adv Drug Deliv Rev. 2010;62:394–407. doi: 10.1016/j.addr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 13.de la Fuente M, Csaba N, Garcia-Fuentes M, Alonso MJ. Nanoparticles as protein and gene carriers to mucosal surfaces. Nanomedicine (Lond) 2008;3:845–57. doi: 10.2217/17435889.3.6.845. [DOI] [PubMed] [Google Scholar]
- 14.Cruz LJ, Tacken PJ, Fokkink R, Figdor CG. The influence of PEG chain length and targeting moiety on antibody-mediated delivery of nanoparticle vaccines to human dendritic cells. Biomaterials. 2011;32:6791–803. doi: 10.1016/j.biomaterials.2011.04.082. [DOI] [PubMed] [Google Scholar]
- 15.Yu D, Wang A, Huang H, Chen Y. PEG-PBLG nanoparticle-mediated HSV-TK/GCV gene therapy for oral squamous cell carcinoma. Nanomedicine (Lond) 2008;3:813–21. doi: 10.2217/17435889.3.6.813. [DOI] [PubMed] [Google Scholar]
- 16.Wattendorf U, Merkle HP. PEGylation as a tool for the biomedical engineering of surface modified microparticles. J Pharm Sci. 2008;97:4655–69. doi: 10.1002/jps.21350. [DOI] [PubMed] [Google Scholar]
- 17.Bandyopadhyay A, Fine RL, Demento S, Bockenstedt LK, Fahmy TM. The impact of nanoparticle ligand density on dendritic-cell targeted vaccines. Biomaterials. 2011;32:3094–105. doi: 10.1016/j.biomaterials.2010.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahmy TM, Demento SL, Caplan MJ, Mellman I, Saltzman WM. Design opportunities for actively targeted nanoparticle vaccines. Nanomedicine (Lond) 2008;3:343–55. doi: 10.2217/17435889.3.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Hagan DT, Valiante NM. Recent advances in the discovery and delivery of vaccine adjuvants. Nat Rev Drug Discov. 2003;2:727–35. doi: 10.1038/nrd1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirby DJ, Rosenkrands I, Agger EM, Andersen P, Coombes AG, Perrie Y. Liposomes act as stronger sub-unit vaccine adjuvants when compared to microspheres. J Drug Target. 2008;16:543–54. doi: 10.1080/10611860802228558. [DOI] [PubMed] [Google Scholar]
- 21.Faisal SM, Yan W, McDonough SP, Chang YF. Leptospira immunoglobulin-like protein A variable region (LigAvar) incorporated in liposomes and PLGA microspheres produces a robust immune response correlating to protective immunity. Vaccine. 2009;27:378–87. doi: 10.1016/j.vaccine.2008.10.089. [DOI] [PubMed] [Google Scholar]
- 22.Mohanan D, Slutter B, Henriksen-Lacey M, Jiskoot W, Bouwstra JA, Perrie Y, et al. Administration routes affect the quality of immune responses: a cross-sectional evaluation of particulate antigen-delivery systems. J Control Release. 2010;147:342–9. doi: 10.1016/j.jconrel.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Allison AG, Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974;252:252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- 24.Altman A, Dixon FJ. Immunomodifiers in vaccines. Adv Vet Sci Comp Med. 1989;33:301–43. doi: 10.1016/b978-0-12-039233-9.50013-5. [DOI] [PubMed] [Google Scholar]
- 25.O’Hagan DT, Rahman D, McGee JP, Jeffery H, Davies MC, Williams P, et al. Biodegradable microparticles as controlled release antigen delivery systems. Immunology. 1991;73:239–42. [PMC free article] [PubMed] [Google Scholar]
- 26.Dudani R, Chapdelaine Y, Faassen Hv, Smith DK, Shen H, Krishnan L, et al. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J Immunol. 2002;168:5737–45. doi: 10.4049/jimmunol.168.11.5737. [DOI] [PubMed] [Google Scholar]
- 27.Demento SL, Bonafe N, Cui W, Kaech SM, Caplan MJ, Fikrig E, et al. TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis. J Immunol. 2010;185:2989–97. doi: 10.4049/jimmunol.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–93. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demento SL, Siefert AL, Bandyopadhyay A, Sharp FA, Fahmy TM. Pathogen-associated molecular patterns on biomaterials: a paradigm for engineering new vaccines. Trends Biotechnol. 2011;29:294–306. doi: 10.1016/j.tibtech.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–63. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev. 2010;236:151–66. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Germain RN. Vaccines and the future of human immunology. Immunity. 2010;33:441–50. doi: 10.1016/j.immuni.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–63. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 35.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–9. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 36.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;131:509–17. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ochsenbein AF, Karrer U, Klenerman P, Althage A, Ciurea A, Shen H, et al. A comparison of T cell memory against the same antigen induced by virus versus intracellular bacteria. Proc Natl Acad Sci U S A. 1999;96:9293–8. doi: 10.1073/pnas.96.16.9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–56. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 40.Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–87. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–95. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–7. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 43.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 44.Krishnan L, Dicaire CJ, Patel GB, Sprott GD. Archaeosome vaccine adjuvants induce strong humoral, cell-mediated, and memory responses: comparison to conventional liposomes and alum. Infect Immun. 2000;68:54–63. doi: 10.1128/iai.68.1.54-63.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeong JM, Chung YC, Hwang JH. Enhanced adjuvantic property of polymerized liposome as compared to a phospholipid liposome. J Biotechnol. 2002;94:255–63. doi: 10.1016/s0168-1656(01)00430-8. [DOI] [PubMed] [Google Scholar]
- 46.Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10:243–51. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen S, Bernstein H, Hewes C, Chow M, Langer R. The pharmacokinetics of, and humoral responses to, antigen delivered by microencapsulated liposomes. Proc Natl Acad Sci U S A. 1991;88:10440–4. doi: 10.1073/pnas.88.23.10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richards RL, Rao M, Vancott TC, Matyas GR, Birx DL, Alving CR. Liposome-stabilized oil-in-water emulsions as adjuvants: increased emulsion stability promotes induction of cytotoxic T lymphocytes against an HIV envelope antigen. Immunol Cell Biol. 2004;82:531–8. doi: 10.1111/j.0818-9641.2004.01282.x. [DOI] [PubMed] [Google Scholar]
- 49.Harding CV, Collins DS, Kanagawa O, Unanue ER. Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol. 1991;147:2860–3. [PubMed] [Google Scholar]