Figure 1.
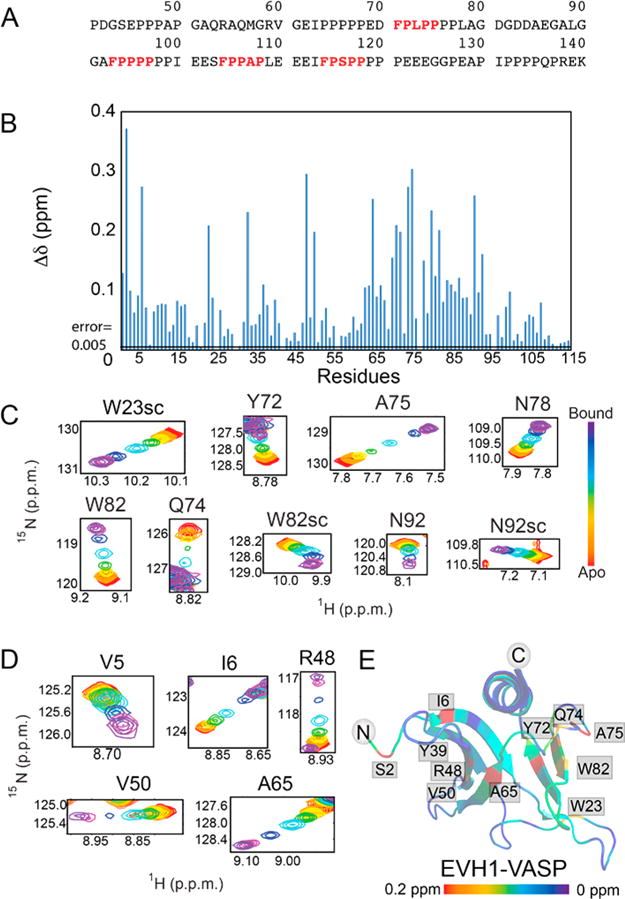
Zyxin41–140 titration provides evidence of a secondary binding site in the EVH1 domain of VASP. (A) Amino acid sequence of the Zyxin41–140 construct, highlighting the four VASP binding motifs (red, bold type). (B) Composite chemical shift changes in [15N]EVH1 induced by addition of Zyxin41–140, calculated using the equation . Extracted regions of overlays of 15N–1H HSQC spectra of [15N]EVH1 titrated with Zyxin41–140 show trajectories for residues in (C) the canonical binding site and (D) a novel secondary site, all of which show maximum Δδ values in excess of 10 times the error in Δδ (determined to be 0.005). (E) Chemical shift changes mapped onto the structure of VASP EVH1 (Protein Data Bank entry 1EGX) show that Zyxin41–140 induces chemical shift perturbations for residues in the primary binding site (far right) but also for residues on the opposite face of the EVH1 domain (far left). Red denotes the largest chemical shift perturbation and purple no perturbation.
