Abstract
People are living longer. On the whole, they have healthier lives and many of the problems previously seen at a younger age now appear in their later years. Kidneys, like other organs, age, and kidney disease in the aged is a prime example. In the United Kingdom, as in other developed countries, the prevalence of end stage kidney disease is highest in the 70- to 79-year-old age group. There are many older people who require renal replacement and are now considered for dialysis. While older patients with end-stage renal disease invariably aspire for a better quality of life, this needs a specialized approach and management. In January 2017, the Royal Society of Medicine held a seminar in London on “Kidney Disease in Older People” with presentations from a multidisciplinary body of experts speaking on various aspects of kidney problems in this age group and its management. The objectives were to increase awareness and improve the understanding of nephrology in the context of geriatric medicine but also geriatrics in its interface with nephrology, especially in the area of chronic kidney disease.
Keywords: clinical geriatrics, kidney disease, renal replacement therapy, decision making, gerontology, aging kidneys
Introduction
People are living longer. On the whole, they have healthier lives and many of the problems previously seen at a younger age now appear in their later years. Kidneys, like other organs, age (Glassock, Denic, & Rule, 2017), and kidney disease in the aged is a prime example. In the United Kingdom, as in other developed countries, the prevalence of end stage kidney disease is highest in the 70- to 79-year-old age group. There are many older people who require renal replacement and are now considered for dialysis. While older patients with end-stage renal disease invariably aspire for a better quality of life, this needs a specialized approach and management.
In January 2017, the Royal Society of Medicine held a seminar in London on “Kidney Disease in Older People” with presentations from a multidisciplinary body of experts speaking on various aspects of kidney problems in this age group and its management.
The objectives were to increase awareness and improve the understanding of nephrology in the context of geriatric medicine but also geriatrics in its interface with Nephrology, especially in the area of chronic kidney disease.
The Aging Kidneys
Like any other organ, aging affects the kidney. Function slowly declines from the fourth decade, although this does not manifest clinically in normal circumstances. In fact, senescent kidneys, when not affected by disease, are able to maintain homeostatic equilibrium, but this fine balance may be rapidly lost when the individual is placed under stress. It is this loss of physiological reserve which is important to be aware of when managing older patients and can impact on many aspects of their care, from which drugs to prescribe to how fluid and electrolytes are best replaced.
This concise review provides an overview of the anatomical and physiological changes the kidneys undergo with aging.
Anatomical Changes With Age
Starting at birth, renal weight steadily increases achieving a peak of 250 to 270 g by the fourth decade. Thereafter, it gradually declines reaching 180 to 200 g by the seventh to eighth decade. At autopsy, approximately 50% of kidneys show a fine granular or mottled surface, and up to 14% are grossly scarred. Senescent scarring is widely distributed over the whole surface. This is in contrast to pyelonephritic scarring which is usually only found at the poles and causes calyceal deformity, absent in purely aged kidneys. Estimates of the degree of age-related volume loss are variable and depend on the modality used. Whereas postmortem studies describe the loss as 20% between the ages of 50 and 80 years, CT scan shows even greater change with a decrease in transverse area of 30% to 33% after the age of 70 years. The loss of renal mass predominantly affects the cortex with relative medullary sparing. This is associated with an increase in the amount of fat deposition in the renal sinuses which at postmortem can account for 17% of the renal mass. Approximately one-half of all people above 40 years of age have one or more simple cysts in the kidneys. These degenerative cysts are typically unilocular and contain clear fluid resembling that of interstitial fluid. Simple cysts are thought to arise from dilated tubules, glomeruli, or even tubular diverticula which become cut off from the tubule.
There is a general consensus that the number of glomeruli decreases with age. Histologically, the glomerular basement membrane becomes thickened and the mesangium increases. The cross-sectional area of the glomerulus also decreases. The capillary tuft in the center of the glomerulus loses complexity leading to a reduction in filtering surface. The filtering membrane itself becomes wrinkled and more permeable to large molecules such as proteins which then appear in the urine. Glomeruli are lost by the process of glomerulosclerosis where the cells degenerate, the capillary tuft volume decreases, and there is an associated accumulation of proteinaceous material. The prevalence of glomerulosclerosis increases with age, from 2.7% at the 18 to 29 years to 73% in ages >70 years (Rule et al., 2010). Furthermore, the percentage of sclerosed glomeruli increases with age and is higher in men. By the eighth decade, it is calculated that up to 30% of glomeruli are sclerotic (Smith, Hoy, & Cobb, 1989).
There is a decrease in number of tubules which degenerate and atrophy with age. Individual tubules show reduced length, decrease in volume, and increased diverticula. At a cellular level, there may be wrinkling and thinning of the tubular basement membrane, simplification of the tubular epithelium and dilatation with regular hyaline casts in the lumen. Therefore, the whole of the nephron undergoes age-related change. The interstitium, comprising the extravascular, internephronal area of the kidney parenchyma, also undergoes changes with aging; the proportion of interstitial tissue made up of glomeruli and tubules decreases fourfold, and the volume is now increasingly made up of areas of scarring, fibrosis, and tubular atrophy.
The renal vasculature also undergoes important changes with aging. In the small interlobular arteries, progressive thickening of the intima is noted at the expense of the media. This process, termed intimal fibroplasias, is patchy and may be the underlying cause of the heterogeneous loss of nephrons. The arteries themselves become distorted and spiraled. In smaller afferent arterioles, there is thickening of the intimal layer due to deposition of hyaline and collagen fibers (hyaline arteriosclerosis).
Total renal blood flow is preserved up until the fourth decade of life, but thereafter a progressive decline of approximately 10% per decade occurs. The decrease in renal blood flow is most profound in the cortex with relative sparing of the deeper medullary areas. At a glomerular level, the reduced blood flow leads to a reduction in glomerular filtration. There is also evidence that autoregulation may be lost or altered with aging and that both the sensitivity and responsiveness of the renal arterioles may be affected. Table 1 provides a summary of the changes that occur with aging.
Table 1.
Morphological Changes That Occur in the Aging Kidney.
| Structure | Individual component | Change |
|---|---|---|
| Gross anatomy | Renal mass | ↓ |
| Scarring | ↑ | |
| Cysts | ↑ | |
| Vascular | Vascular resistance | ↑ |
| Intimal thickness | ↑ | |
| Total renal blood flow | ↓ | |
| Homeostatic responsiveness | ↓ | |
| Tortuosity | ↑ | |
| Glomerular and interstitial tissue | Number of glomeruli | ↓ |
| Cross-sectional area of glomeruli | ↓ |
Physiological Changes With Age
Studies, both cross sectional and longitudinal, have been consistent in demonstrating a steady decline in glomerular filtration rate (GFR) with age starting from the fourth decade with an accelerated rate of decline by the seventh decade (Lindeman, Tobin, & Shock, 1985; Rowe, Andres, Tobin, Norris, & Shock, 1976; Wesson, 1969). The average decline in GFR is 0.96 mL/min/year or about 10 mL/min/decade with creatinine clearance (CrCl) falling from 140 mL/min/1.73 m2 at age 30 years to about 97 mL/min/1.73 m2 at age 80 years (Rowe et al., 1976. It should be noted that lower rate of decline of 0.75 mL/min/year has been reported in the Baltimore longitudinal study of aging (Lindeman et al., 1985), as has higher rate (Hemmelgarn et al., 2006). Although not a universal process (Lindeman et al., 1985), this downward slope in GFR appears to be a normal process of aging and, importantly, independent of hypertension or other comorbid conditions. The decline is related to progressive loss of glomeruli through global glomerulosclerosis and changes in the renal vascular tree, perhaps related to the effect of oxidative stress and telomere shortening, or linked to an action of angiotensin II.
Functional changes are also seen in the tubules. In the proximal tubule, the maximum rate of reabsorption (Tm) for some substances is decreased. For example, the Tm of glucose diminishes in tandem with GFR. The proximal tubule also has an important metabolic function such as the removal of insulin. Insulin is filtered by the glomerulus and then is absorbed by renal tubular cells which then degrade it. As this function declines with age, insulin clearance decreases.
The kinetics of sodium handling is probably more relevant. With age, the ability of the ascending limb to reabsorb sodium is decreased, leading to a higher osmotic load on the distal sections of the tubule which can only reabsorb approximately 3% of the sodium in the glomerular filtrate. This may lead to a state of overall sodium loss. In one study, the ability to conserve sodium following acute dietary salt restriction in older individuals, assessed by determining the half time taken for proportionate reduction in sodium excretion, was at almost twice that of younger people (31 vs. 17 hr; Epstein & Hollenberg, 1976). Conversely, due to the generally lower GFR of elderly subjects, sodium loading takes longer to be cleared so normal elderly subjects can be described as “salt sensitive” (Luft, Grim, Willis, Higgins, & Weinberger, 1977).
The sluggish adaptive response in older people to abrupt changes in salt intake may have major consequences, for example, following surgery or acute illness, resulting in reduction in extracellular fluid compartment and effective circulatory volume. Not only is excretory capacity for sodium impaired, but there is also a circadian variation in that older people excrete sodium at higher rates at night compared with younger persons (Kirkland, Lye, Levy, & Banerjee, 1983).
Renal senescence also affects potassium handling. In older subjects, the basal urinary secretion of potassium is decreased, predisposing to the occurrence of drug-induced hyperkalemia (Zhou et al., 2008). This has been attributed to a relatively low serum aldosterone and aldosterone resistance in old age.
Alongside the progressive age-related decrease in aldosterone level (both basal and in response to upright posture and volume depletion), the activity of the renin–angiotensin system appears to decline in both baseline and stimulated states. In the stimulated state, it is estimated that plasma renin activity in older people is 40% to 60% lower than in younger population (Crane & Harris, 1976; Weidmann, De Myttenaere-Bursztein, Maxwell, & de Lima, 1975). The sluggish renin–aldosterone response to acute stimuli may explain the reduced capacity for sodium retention and reduced potassium excretion.
The ability to concentrate urine also declines with age (Rowe, Shock, & DeFronzo, 1976). The reasons for this are still not entirely clear. Initially, this was attributed to the decline in GFR, but studies have failed to confirm this. Other studies postulated that it is due to increased medullary blood flow leading to “washout” of the medullary concentrating gradient. In support is that older subjects have been shown to have a higher fractional excretion rate of urea which is an important contributor to maintaining this gradient.
The declining ability to concentrate urine and conserve water may in certain cases accentuate dehydration, especially in summer months during unexpected sudden surges in temperature like the occurrence of a heat wave. This is more marked in patients with preexisting diseases or those on diuretics. At the other end of the spectrum, the diminished ability of the senescent kidney to produce dilute urine in response to water or saline load may become problematic in older people leading to fluid overload, and clinically relevant in those with underlying compromised cardiac function.
These physiologic changes lead to a greater tendency to both hypernatremia and hyponatremia in older people. They often render the course of acute illness to become complicated by derangements in fluid and electrolyte homeostasis resulting in delayed recovery. Furthermore, the blunted aldosterone response in association with a reduced GFR in older people increases the risk of hyperkalemia. The problem is further compounded by certain drugs that possess the potential to impair potassium handling like potassium sparing diuretics, NSAIDS, and beta blockers, commonly prescribed in older people.
Other changes that occur in the function of the kidneys with aging are summarized in Table 2.
Table 2.
Summary of the Physiological Changes That Occur in the Kidney With Aging.
| Function | Component | Change |
|---|---|---|
| Glomeruli | Membrane permeability | ↑ |
| Glomerular filtration rate | ↓ | |
| Tubulointerstitial | Electrolyte homeostasis | ↓ |
| Metabolic functions | ↓ | |
| Response to dehydration | ↓ | |
| Fluid balance | Urine concentrating ability | ↓ |
| Vasopressin secretion | ↑ | |
| Total body water | ↓ | |
| Homeostatic responsiveness | ↓ | |
| Acid-base | Homeostatic responsiveness | ↓ |
| Renin–angiotensin | Activity | ↓ |
| Postural response | ↓ | |
| Erythropoiesis | Incidence of anemia | ↑ |
| Erythropoietin level | ↑ | |
| Vitamin D | α-hydroxylation | ↓ |
| Renal metabolism | ↑ |
In conclusion, homeostasis in older people can be maintained in a state of health but response to stress is blunted significantly. Sudden fluid and electrolyte depletion cannot be tolerated as well in older subjects and is much more likely to lead to serious complications such as hypotension and collapse.
Chronic Kidney Disease in Older People: Assessment of Renal Function
Chronic kidney disease (CKD) is common particularly in older people. The introduction of glomerular filtration rate (GFR) estimating equations for the evaluation of kidney function and the development of a classification framework that uses a GFR of <60 mL/min/1.73 m2 as a threshold for diagnosis of CKD has raised awareness of kidney disease in older people but also stimulated debate as to its significance. Population data from the United States show that more than 40% of those aged above 70 years have an estimated GFR (eGFR) less than 60 mL/min/1.73 m2 (Coresh et al., 2007), leading some to contend that CKD is an example of “overdiagnosis.”
CKD is defined by structural or functional abnormalities of the kidney, with or without the presence of a reduced GFR, irrespective of the cause. In 2002, the Kidney Disease Outcomes Quality Initiative (KDOQI) launched a five-stage framework to help classify CKD, in an attempt to simplify the diagnosis. In 2012, the classification was revised by Kidney Disease Improving Global Outcomes (KDIGO) to include both eGFR and albuminuria in the staging. These classification systems have been universally adopted by health professionals to identify people with CKD, but have not been without controversy, particularly in their application to older people.
Estimating GFR
The GFR estimating equations in common use are based on serum creatinine concentration. Unlike the traditional Cockcroft–Gault equation, the four-variable Modification of Diet in Renal Disease (MDRD) equation, first described in 1999, is normalized for body surface area and does not require knowledge of the individual’s weight. This facilitated the routine reporting of eGFR by pathology laboratories, which in the United Kingdom began in 2005 and has transformed the management of CKD. A major limitation of the MDRD study equation is that it underestimates kidney function at or above a GFR of 60 mL/min/1.73 m2 leading to an overestimation of CKD prevalence (Rule et al., 2006).
In 2009, the CKD-Epidemiology Collaboration (CKD-EPI) equation was proposed as a more accurate equation to estimate GFR. In the 2014 guideline on CKD (CG182), the National Institute of Health and Care Excellence (NICE) recommended that clinical laboratories should use CKD-EPI to estimate GFR, using creatinine assays with calibration traceable to standardized reference material. The use of CKD-EPI reduces the prevalence of CKD stages 3 to 5 in the general population and improves risk prediction (Matsushita et al., 2012), although it results in an increased prevalence of eGFR <60 mL/min/1.73 m2 in older people (Levey et al., 2009). As with the MDRD study equation, CKD-EPI is still limited by the use of serum creatinine as a filtration marker.
The Cockcroft–Gault equation to estimate creatinine clearance has largely been superseded by MDRD and CKD-EPI. However, most published studies for drug dosing are based on Cockcroft–Gault. In older people who may have reduced muscle mass, the use of creatinine-based equations can lead to overestimation of renal function. Cockcroft–Gault will result in a more conservative estimation of renal function in this group but at the risk of under dosing. While most drugs can be safely dosed on the basis of the eGFR, UK Medicines Information recommend that dose regimens based on creatinine clearance calculated by the Cockcroft–Gault equation should be used for potentially toxic drugs with a narrow therapeutic index, together with monitoring of plasma-drug concentrations and clinical response.
Cystatin C is a newer biomarker of kidney function and potentially an improved method for estimation of GFR. It is a nonglycosylated, basic protein that is produced by all nucleated cells, freely filtered by the glomerulus and then reabsorbed by the tubular epithelial cells. In a large, multicenter population-based cohort study, cystatin C improved accuracy in identifying patients with CKD and distinguished patients with high-risk CKD (Peralta et al., 2011). NICE CG182 recommended that eGFRcystatin C should be considered at initial diagnosis to confirm or rule out CKD in people with a creatinine-based eGFR of 45 to 59 mL/min/1.73 m2 sustained for at least 90 days and no albuminuria or other marker of kidney disease.
Changes in Kidney Function With Age
Although the incidence of pathological diseases within the kidney, such as diabetes, atherosclerosis, and vasculitis, is greater in the elderly population, structural and functional changes in the kidney may be seen with aging, even without the presence of a specific underlying kidney disease (Glassock, 2014). The point has been made that disease labeling of older people may result in unnecessary intervention and treatments leading to increased anxiety and reduced quality of life for the patient and increased burden of work and cost for health professionals. In this context, some have called for the introduction of age-specific reference ranges, arguing that
the lack of an age calibration in the KDIGO-2012 framework for the diagnosis of CKD is an inherent and potentially serious weakness that may lead to substantial over-diagnosis of CKD in otherwise healthy populations of older and elderly subjects and its under-diagnosis in younger individuals. (Glassock, 2014)
So is decline of GFR simply due to organ senescence and a normal part of aging? In the population-based cross-sectional Nijmegen Biomedical Study, conducted in the Netherlands, the 5th, 50th, and 95th percentiles of eGFR in nondiseased Caucasian males aged 75 to 79 years was 45, 70, and 91 mL/min/1.73 m2, respectively, and 45, 61, and 82 mL/min/1.73 m2 in nondiseased Caucasian females aged 75 to 79 years (Wetzels, Kiemeney, Swinkels, Willems, & den Heijer, 2007). The authors concluded that a GFR of 60 mL/min/1.73 m2 was within the normal reference range for men >60 and women >50 years and should not be used to define a diseased population.
However, the Baltimore Longitudinal Study of Aging, published more than 30 years ago, suggests that a decline in renal function is not an inevitable consequence of aging. In this study, the creatinine clearances of 254 “normal” men, aged 22 to 97 years, measured over 8 to 14 years, showed that one third of all subjects had no absolute decrease in renal function when studied longitudinally. In fact, two individuals actually showed a statistically significant increase (p < .05) in creatinine clearance with age (Lindeman et al., 1985).
The interpretation of these data is still the subject of debate. The length of follow-up may have been too short in some subjects and the number of measurements taken too few to be truly confident that there was no statistically significant decline in renal function in more patients. Participants with nonproteinuric diabetes were included in this “normal” group, raising the possibility of glomerular hyperfiltration, the earliest clinical manifestation of classical diabetic kidney disease as a confounding factor (Glassock, 2014).
Significance of CKD in Older People
Given this controversy, it is appropriate to next consider the theoretical and practical implications of a CKD diagnosis. It is clear that the most important reason to identify older individuals with CKD is not to prevent ESRD. Older patients with CKD have a far higher risk of death from other causes than progression to ESRD; only at eGFR levels <15 mL/min/1.73 m2 does risk for ESRD exceed risk for death among those aged 65 to 84 years (O’Hare et al., 2007). However, we know from the CKD Prognosis Consortium’s large-scale and robust epidemiological data that in both high-risk (van der Velde et al., 2011) and general population (Matsushita et al., 2010) cohorts, patients at all stages of CKD are at an increased risk of both cardiovascular and all-cause mortality. In the specific case of the elderly the risk of mortality is increased at an eGFR below 60 mL/min/1.73 m2 (Hallan et al., 2012), the threshold currently used to define CKD GFR category 3, arguing against the need for age-specific CKD diagnosis and staging. It is also important to appreciate that while the relative risks of adverse outcomes associated with low GFR in the elderly are lower than those in younger age groups, the attributable risks are substantially greater.
There are other factors that underscore the importance of diagnosing CKD in older people. Data from the Baltimore Longitudinal Study of Aging have shown that cognitive impairment is associated with a decline in GFR (Seliger, Wendell, Waldstein, Ferrucci, & Zonderman, 2015). In a cross-sectional analysis of the Third National Health and Nutrition Examination Survey (NHANES III), moderate to severe kidney disease was independently associated with a twofold increase in history of hip fracture (Nickolas, McMahon, & Shane, 2006).
The presence of preexisting CKD, at any stage, is a powerful risk for the development of acute kidney injury (AKI), which is associated with significant morbidity and mortality. Mortality for patients with severe AKI requiring renal replacement therapy is around 60%. Older age is associated with higher AKI risk at all stages of CKD compared with younger age groups, which was attenuated at lower levels of eGFR (Grams et al., 2015). The NICE guideline on AKI (CG169) indicates that consideration should be given to short-term cessation of renin–angiotensin blocking drugs in people with diarrhea, vomiting, or sepsis until their clinical condition has improved and stabilized.
A CKD diagnosis has a number of other implications for medicines management. Nonsteroidal anti-inflammatory drugs, which may be purchased over the counter in the UK, have well-documented effects on renal function. People with CKD have an increased susceptibility to adverse drug reactions, which is compounded by polypharmacy and frailty in the elderly. The doses of a number of commonly prescribed medications (e.g., Gabapentin, opioids, and some newer oral hypoglycemic agents) should be adjusted even with relatively minor reductions in kidney function.
Conclusion
Although CKD in older people is common and diagnosable using eGFR-estimating equations validated in this patient group, there continues to be skepticism from some quarters about its importance, together with a reluctance to inform patients of the diagnosis. Given the clear relationship between CKD and harm, which may be avoidable, the diagnosis of CKD, and the communication of this diagnosis to the patient, irrespective of age, in a sensitive manner tailored to individual circumstances, and should be seen as an important vehicle for health care professionals to encourage better care and self-management in this vulnerable patient population.
Frailty and Cognitive Impairment in End-Stage Renal Disease
As the dialysis population ages, geriatric syndromes including frailty and cognitive impairment are increasingly common. Frailty is increasingly prevalent with declining renal function and affects up 70% of the dialysis population. It is associated with increased mortality, hospitalization, and disability. Cognitive impairment also affects a similar proportion of patients, yet remains underrecognized. It has a cerebrovascular pathogenetic basis. Executive function is the predominant cognitive domain affected. Cognitive impairment is associated with poorer survival, increased use of staff resources, and hospital stay in patients with end-stage renal disease (ESRD).
These findings highlight the need to screen for geriatric syndromes in older patients with ESRD. Research studies are however required to identify valid screening tools as well as strategies to limit the impact of these syndromes on renal patients.
People with ESRD are increasingly older, consistent with demographic trends in the general population. In 2014, renal registry data from the United kingdom showed that the median age for the prevalent dialysis population was 59 years, with peak prevalence between 75 and 79 years of age (MacNeill, Casula, Shaw, & Castledine, 2016). These findings are consistent with reports from other developed countries. The older patient with ESRD poses additional challenges to clinical care. This is due to the prevalence of geriatric syndromes including frailty, falls, multimorbidity, and cognitive impairment.
Frailty in ESRD
Frailty has been defined as a distinctive health state associated with the aging process, characterized by a multisystem loss of reserve and consequent impaired response to minor stressful events. Frailty is a difficult construct to operationalize. There are two widely accepted models for defining the construct of frailty.
The phenotypic model using the Fried index defines frailty as the presence of three or more of the following: recent unintentional weight loss, exhaustion, low grip strength, slow walk speed, low physical activity. This criterion-based definition was derived from a secondary analysis of data from the Cardiovascular Health Study (Fried et al., 2001).
The cumulative deficit model assesses frailty in continuum, defining frailty as proportion of accumulated deficits out of 70 possible clinical variables. Variables include comorbidities, symptoms, and blood tests (Rockwood et al., 2005). This model is thought to correlate better with the aging process.
Prevalence of frailty in ESRD
Chronic kidney disease (CKD) has been shown to be an independent risk factor for frailty (Dalrymple et al., 2013). The relationship between CKD and frailty is a graded one. The incidence and prevalence of frailty has been shown to increase as renal function deteriorates. Frailty has been reported in up to 70% of the dialysis population (Johansen, Chertow, Jin, & Kutner, 2007). Although frailty is associated with aging, it has been shown to be common even in young dialysis patients, highlighting renal disease as a state of accelerated aging. The reported prevalence of frailty also varies depending on the assessment tool used.
Frailty and clinical outcomes in ESRD
Frailty has been shown to be associated with adverse outcomes in patients with ESRD, irrespective of chronological age (Johansen et al., 2007). As in the general population, frailty in dialysis patients is associated with a higher risk of death, hospitalization, falls, and disability (Johansen et al., 2007). It has also been shown to be associated with a higher estimated GFR at the start of the dialysis (Bao, Dalrymple, Chertow, Kaysen, & Johansen, 2012), suggesting that symptoms attributed to uremia may actually reflect frailty in some patients. Yet the initiation of dialysis has been associated with functional decline in older people with ESRD (Kurella Tamura et al., 2009). While they are not synonymous, physical function and frailty are closely related. Thus, the effects of frailty are unlikely to be reversed with the initiation of dialysis. Frailty has also been associated with poor quality of life outcomes, in older dependent patients on dialysis, irrespective of the dialysis modality. In a study evaluating QoL outcomes in older dependent patients on dialysis, QoL measures did not differ between assisted peritoneal dialysis (PD) patients and those on hemodialysis (HD). However, the frailty score was predominantly associated with adverse QoL outcomes (Iyasere et al., 2016).
Cognitive Impairment and ESRD
The interaction between the brain and dialysis has evolved over time, with advances in dialytic process. Over four decades ago, dementia dialytica was described as an organic brain syndrome characterized by progressive dementia, psychotic behaviors, dysarthria, dyspraxia, and seizures, associated with death within 6 months. This condition, often associated with aluminum toxicity, now rarely exists.
Cognitive impairment however still exists in patients with CKD, with increasing prevalence as renal function declines. In the HD population, prevalence rates of up to 70% have been reported. In this particular study, only 3% had a clinical diagnosis of cognitive impairment (Murray et al., 2006). Similar prevalence rates have been reported in the PD population.
Pathogenesis of cognitive impairment in ESRD
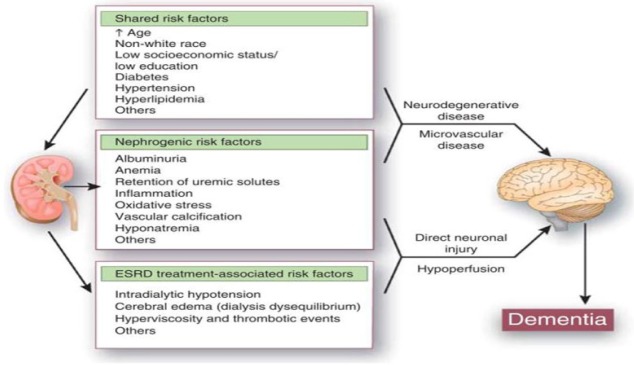
Cognitive impairment in renal disease is thought to be the result of a complex interplay between vascular, nephrogenic, and dialytic risk factors (Figure 1). Cerebrovascular disease is considered to be the basis for cognitive impairment in renal patients. Executive function is the predominant cognitive domain affected in renal patients. The magnetic resonance imaging (MRI) findings of silent cerebral infarcts, atrophy, micro bleeds, and subclinical white matter changes seen in renal patients are not dissimilar from those seen in patients with vascular dementia.
Figure 1.
Potential pathogenetic mechanisms for cognitive impairment in ESRD.
Source. Reproduced with permission from Kurella Tamura and Yaffe (2011).
Note. ESRD = end-stage renal disease.
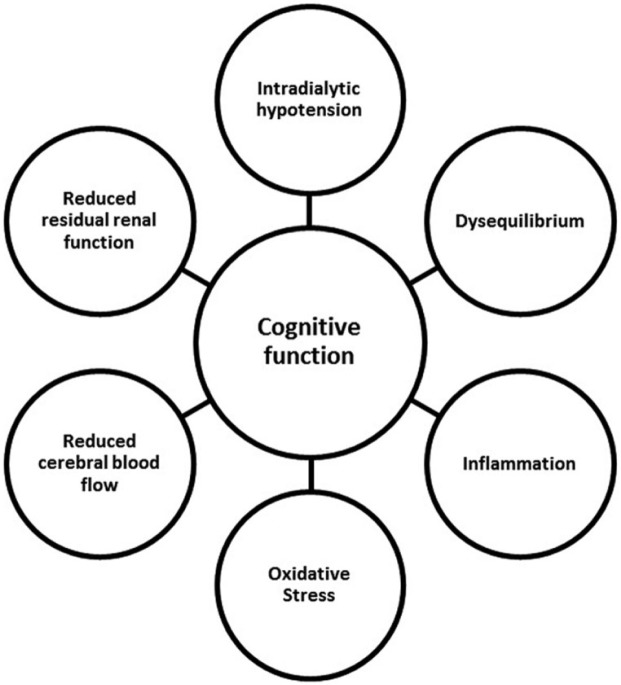
Cognitive dysfunction may also be exacerbated by the dialysis process itself. Cognitive function has been shown to fluctuate during a HD session. There is also emerging evidence that cognitive function declines with the initiation of dialysis (Kurella Tamura et al., 2017). The mechanisms by which dialysis affects cognitive function remain poorly elucidated. Some of the purported mechanisms are shown in Figure 2. Dialysis modality may also be a risk factor for cognitive decline in patients with ESRD. A large retrospective study suggests that the cumulative incidence of dementia (predominantly vascular) is higher in HD patients when compared with those on PD (Wolfgram, Szabo, Murray, & Whittle, 2015). Prospectively, there is some evidence that cognitive function declines more rapidly in HD patients compared with those on PD.
Figure 2.
Potential dialytic effects on cognitive function.
Source. Reproduced with permission from Iyasere and Brown (2017).
Cognitive impairment and outcomes in ESRD
As in the general population, dialysis patients with dementia have been shown to have a higher risk of death compared with those without dementia. Cognitive impairment has been also independently associated with poorer survival after 7 years in dialysis patients (Griva et al., 2010). It has also been associated with increased hospital stay and increased care time after dialysis. Pilot studies also suggest that cognitive impairment potentially influences decision making capacity in patients with ESRD.
Screening for Frailty and Cognitive Impairment in Patients With ESRD
There is now robust evidence showing that frailty and cognitive impairment are common in ESRD. Yet they are not always recognized in routine nephrology practice. This emphasizes the need to screen for geriatric syndromes, especially in older people with ESRD. The recognition of frailty and cognitive decline are potential triggers for discussing renal replacement options, including active nondialysis care. They may also serve as prompts for advance care planning in patients with ESRD. The use of appropriate screening tools in often time-constrained clinical practice can be challenging.
Frailty has been evaluated by several assessment tools, in research studies of patients with ESRD. It has been predominantly evaluated by modified versions of the Fried index whereby the original criteria pertaining to physical function have been replaced with self-reported measures. These measures, while predictive of adverse outcomes, have not been extensively validated in the renal population. Further validation studies are required in patients with ESRD.
Validation studies are also lacking for cognitive assessment tools in renal patients. The ideal cognitive assessment tool would be sensitive to changes in executive function, as the predominant domain affected in renal patients. The validity of the Montreal Cognitive Assessment (MoCA) has been tested in a small study of 43 HD patients and 42 healthy controls. It has the advantage over other screening tools (such as the mini-mental state examination [MMSE]), as it assesses executive function. It has been shown to be sensitive to changes in cognition during dialysis. Validated against a neuropsychological battery, a MoCA score less than 24 has been suggested to be indicative of cognitive impairment (Tiffin-Richards et al., 2014). This contrasts with the cutoff of 26 in the general population.
Clinical interventions for frailty and cognitive impairment in ESRD
There is a need for interventions to prevent or delay the effects of frailty in patients with ESRD. A Comprehensive Geriatric Assessment (CGA) is one such intervention that has been used in the general population. The feasibility of it use in the renal population warrants further research. The utilization of an exercise program in the dialysis population has been to improve measures of physical function. It remains unclear whether such an intervention is feasible in frail dialysis patients. In terms of cognitive impairment, evidence-based interventions are lacking. Alterations to HD techniques have shown some promise in ameliorating the effects of dialysis on cognition.
Conclusion
Frailty and cognitive impairment are common but under recognized in patients with ESRD. In this population, they do not solely affect older people, but also affect younger patients. Each is independently associated with adverse outcomes, highlighting the need for screening in clinical practice. Further research is required to validate screening tools in the renal population and to identify clinical interventions for the management of these geriatric syndromes.
The Role of the Geriatrician in Management of Older People With End-Stage Renal Disease
Older patients with chronic kidney disease (CKD) and end-stage renal failure (ESRF) have been primarily managed by nephrologists, including the decision to commence renal replacement therapy (RRT). However, the outcome has been disappointing and at variance to that seen in younger patients on RRT. Reflecting on these results various centers have made recommendations for the active involvement of geriatricians in the decision-making process regarding RRT.
As the proportion of older people grows, so does the incidence of CKD and ESRF. Consequently, the last two to three decades has seen a proportional rise in elderly patients commenced on RRT. However, many elderly patients often have multiple comorbidities and specific sociopsychological needs requiring a holistic approach and a thorough multisystem comprehensive assessment. Therefore, an increasingly important role for the geriatrician to play in the management of these patients has become apparent. This review aims to explore this vital role in the management of CKD in the frail elderly patient.
Management of CKD in Older Patients
Management of risk factors and comorbidities
It is well known that the prevalence of CKD increases significantly with age. The decline in GFR is not only due to physiologic changes which occur with organ senescence, but also due to acquired and/or concomitant diseases such as hypertension, diabetes, cardiovascular disease, and obesity. All CKD patients require aggressive cardiovascular risk reduction. Therefore, recognizing and managing these risk factors, for example, tight glycemic control, modification of blood pressure, fluid balance assessment, play an important role in reducing the severity of CKD and progression to ESRF. Geriatricians, as a possible first point of contact, are in prime position to manage these general illnesses. It is also a role for the geriatrician to identify early on, renal disease that may require special treatment, such as underlying glomerulonephritis, vasculitis, and nephrotic-range proteinuria so that appropriate and early referral to the relevant specialty can be made and treatment initiated.
Comorbidities tend to increase both in number and severity with age and worsening CKD. However, it is important to note that it is often a balancing act to maximize treatment of one comorbidity while keeping the adverse effects of treatment on other systems to a minimum. For example, the avoidance of postural hypotension and possible falls when attempting to bring hypertension under control, or when optimizing symptom control in congestive heart failure but without unnecessary compromise to renal function. This risk–benefit intervention is a dynamic process often realized through a holistic approach and assessment. Geriatricians are in an ideal position to assess the older patient holistically and find this balance.
Assessment of physical and cognitive abilities
It has also been shown that cognitive impairment is more common in those with CKD compared with the general population (Anand, Johansen, & Kurella Tamura, 2014). This may be related to the higher prevalence of cardiovascular disease and cerebrovascular small vessel disease in the advanced CKD/ESRF population (Kuriyama et al., 2013).
Compliance with management advice and treatment in ESRF is particularly important. In older people, this can be difficult in the presence of cognitive impairment and may negatively affect the progression of CKD if patient is not appropriately supported by carers and health professionals. Furthermore, studies have shown an associated decline in physical function among older patients with CKD and ESRF (Kurella Tamura et al., 2009). All major domains of function, including ability, performance, leisure time activity, activities of daily living (ADLs), are affected, and the prevalence is higher in patients with eGFR of <40 mL/min compared with eGFR between 40 and 60 mL/min (Plantinga et al., 2011; Roderick et al., 2008). This will impact on the overall functional reserve of the patient and increase social isolation leading to further problems including depression. Through multidisciplinary team work, geriatricians can often play a leading role in orchestrating support networks to be put in place to ensure the patient’s needs from health, physical, cognitive, and social aspects are met.
Assessment and management of frailty
As one would expect, a large number of elderly patients with CKD and ESRF fit the criteria for frailty. Studies evaluating the prevalence of frailty have shown that in a dialysis population aged >60 years, 75% meet the criteria for frailty (Bao et al., 2012). These patients also do particularly badly; frailty is associated with an increased hazard ratio of mortality within the first year of dialysis compared with those that are not frail (Johansen et al., 2007). This suggests that managing and identifying the frail patients are vital in deciding and optimizing treatment options. Frailty as a condition is often best recognized via a comprehensive geriatric assessment and managed by experienced geriatricians through a multidisciplinary approach.
Management of ESRD in Older Patients
Decision regarding management options
Given the complex medical and social needs of older patients, the management strategies of advanced CKD in these patients can be difficult and there is an argument that geriatricians should play a key role in coordinating care (Moranne, Couchoud, & Vigneau, 2012). Management options in ESRF are between RRT (including transplantation) and conservative management. It is now widely accepted that age alone should not be a contraindication to any of the RRT strategies. Instead, functional limitations, dependency levels, and cognition are useful predictors of mortality in elderly patients on dialysis. Therefore, in view of the complex comorbidities and psychosocial factors that are often associated, it is felt that a comprehensive review of each patient entering into ESRF should be undertaken, with the involvement of the patient and carers, to decide which treatment strategy is in the patient’s best interest.
Two main modes of dialysis exist, namely HD and PD. HD remains the most common type of dialysis elderly patients are started on and most studies center around the effect of HD on morbidity and mortality. HD can be achieved in the acute setting with central venous access but more commonly in a planned fashion via arteriovenous fistulae. HD is a time-consuming and demanding treatment, requiring multiple hospital trips weekly in order to undertake dialysis, hence possibly exacerbating or generating a degree of social isolation in a population where there is already a high prevalence of depression. It limits what patients are able to eat, drink, do, and ultimately dictates a lifestyle which the older patient may not be able to adhere to. In addition, due to the volume of fluid shifts during dialysis, it can be poorly tolerated by some patients and they can feel very washed out after session which can negatively impact on daily life.
PD, in contrast, has the benefit of promoting independence from hospital care for self-caring patients and also avoids the issue of dialysis interfering with social life as it can be carried out at home and while away on holiday. In addition, it causes less dramatic shifts in fluid balance so it can be better tolerated by many older patients. For those who are very frail and unable to carry out PD themselves, there are increasing options for assisted PD, whereby carers are trained to provide assistance in the patient’s own home or nursing home. However, PD is not appropriate for all and can be limited by existing medical conditions which act as contraindications for PD such as existing ascites and previous bowel surgery. Furthermore, due to the need to train carers, there is an argument that this can be an additional strain on the carer(s) and the family.
Given the rising number of elderly patients started on dialysis, it is important to ascertain if it confers a survival advantage and whether it justifies the quality of life (QoL) these patients have to live with. An early study (Joly et al., 2003) showed that median survival of octogenarians with ESRF in whom dialysis was started was 28.9 months compared with only 8.9 months on conservative management. This demonstrated a significant prolongation of life and confirmed that selected older patients would benefit from RRT. Another study (Ronsberg, Isles, Simpson, & Prescott, 2005) found that dialysis patients have a mortality rate in between that of lung cancer and MI, showing the effectiveness of RRT in ESRF. Nonetheless, despite the improvement in survival in recent years, the mortality of elderly patients on dialysis remains high. Kurella Tamura (2009) reported the outcome of a cohort of 3,702 elderly patients from nursing homes on dialysis (Kurella Tamura et al., 2009). At mean age of 73.4 years, the mortality at end of 1 year was 58%. Perhaps more importantly only 39% of patients had maintained their predialysis functional status at 3 months which dropped further down to 13% at 1 year. This decline was thought to be independent of age, gender, and functional status trajectory prior to initiation of dialysis.
The substantial decline in functional status on dialysis has been validated by other studies (Jassal, Chiu, & Hladunewich, 2009) and is concerning. Declining functional status may suggest a correlated drop in QoL as these patients lose independence. There have been debates about suitability of RRT for all and whether conservative management may be more appropriate in certain groups of elderly patients. In support of this approach is the study by Chandna et al. (2011) who compared the survival of elderly patients managed conservatively versus those on RRT. The results showed that in those with significant comorbidities, the survival benefits of RRT are likely to be small. In view of these findings and to balance optimal survival benefits while maintaining QoL, recommendations have been made for a comprehensive geriatric assessment to aid the decision-making process. This is best done by a qualified geriatrician or specialist nurse in geriatrics (described below).
Transplantation
Compared with dialysis, transplantation is superior in terms of long-term survival for patients with ESRF. Although age alone is no longer a contraindication to transplantation, the number of elderly patients enlisted is still low. These patients have less chance of receiving a kidney compared with their younger counterparts. Many reasons exist for this difference, including intercurrent illness which affects eligibility for transplant. It has been shown that the survival benefits of transplant versus dialysis is really only evident after 2 years, with a recognized initial rise in mortality in the immediate postoperative period (Segall et al., 2016). This perhaps suggests that those with a particularly short life expectancy would not be appropriate for transplant. A recent study (Lønning et al., 2016) showed that the estimated 5-year survival for octogenarians with a renal transplant was 55%. Undoubtedly, there are risks associated with transplants in the elderly, both in the immediate postoperative care in the longer term. For instance, the altered immune state of the elderly (immunosenescence) means potentially less graft rejection but there is a higher overall risk of infections while immunosuppressed. Transplantation can offer improved longevity and QoL for those eligible; however, patient selection needs to be undertaken with care and should involve both geriatric and renal teams as well as the patients themselves. Stringent multidisciplinary preoperative assessment is important to ensure the appropriate patient is optimized from psychological, physical, and medical perspectives.
Conservative management
Due to the high mortality and morbidity associated with ESRF and the frail elderly patient with multiple comorbidities it affects, sometimes conservative management is more appropriate. Patients with advanced dementia, severe comorbidities, bed-bound status or loss of dexterity of the hands are examples where a conservative approach is a better option. It should be emphasized that a conservative “no dialysis” option is not a “no treatment” option. Conservative management can relieve many symptoms and maximize the person’s health during the remainder of their life. Indeed, as mentioned earlier, studies have suggested conservative management to confer similar survival to RRT in those with a high comorbidity load (Chandna et al., 2011). Conservative treatment should include management of fluid balance, acidosis, anemia, hypertension, as well as pain control, and pruritis. The aim is to optimize QoL.
It is important to emphasize that this decision needs to be undertaken jointly by the geriatric and renal teams, with the patient and their carers.
Palliative care
As ESRF progresses, symptom control often becomes more difficult and involvement of the palliative care team needs to be considered. End of life care and palliation should be patient-centered and planned in advance through discussions with the patient and carers. It is vital to include family members as well.
Proactive care planning can enhance QoL for both patients and their family and help to prevent crises and unscheduled hospital admissions. Palliative care as a management option is explored both in patients on dialysis approaching their end and in those on conservative treatment. A multidisciplinary approach with active involvement from community practice allows the integration of services through various agencies and ensures satisfactory end of life care for patients and relatives.
Conclusion
With an aging population, the incidence of CKD and ESRF is rising. This population of patients is often frail, with multiple comorbidities, and the morbidities and mortality associated with the different treatment options make management of their renal disease complex. Geriatricians, in close cooperation with their nephrologist colleagues, are often best placed to take a leading role in the management of this group. A comprehensive geriatric assessment should be undertaken in each patient to establish the functional, cognitive, medical baseline, and the wishes of the patient. Only then can we formulate the best treatment approach tailored to the patient.
Integrating Geriatrics Into Renal Medicine
UK Renal Registry data show that the prevalence of end-stage kidney disease is highest in the 70- to 79-year-old age group (MacNeill et al., 2016). This plus the aging of the general population is causing a rapid expansion of older patients on dialysis with a 22% increase in patients aged 75 to 84 years and 48% increase in patients ≥85 years between 2009 and 2014. Furthermore, as chronic kidney disease (CKD) is associated with vascular disease, older patients on dialysis have more comorbidities, are more likely to be frail, and have a higher prevalence of cognitive impairment than the general population (Cook & Jassal, 2008; Johansen et al., 2007; McAdams-DeMarco et al., 2015). The cross-sectional analysis of the baseline data of the Frail Elderly Patient Outcomes on Dialysis (FEPOD) study has shown that frailty, and not dialysis modality, is the main factor associated with outcomes of patients on HD and assisted PD (Iyasere et al., 2016). This is not surprising when considering how frailty can impact on patient management (Table 3).
Table 3.
Impact of Frailty on Management of Patients With End-Stage Kidney Disease.
| Frailty aspects | Clinical considerations | Health care challenges |
|---|---|---|
| Cognitive impairment | Degree of impairment and appropriateness of dialysis Progression can be more rapid after starting dialysis Impacts on ability to cope with rigors of dialysis |
Incorporation of cognitive assessment into routine nephrological care Time for complex discussions and advance care planning Adjustment to dialysis |
| Functional impairment | Potential impact of dialysis on functional decline Dependence and optimal dialysis modality Exercise as a preventive strategy Falls and fracture risk Transport requirements |
Liaison with geriatric teams for assessment, falls clinics, and community support Involvement of rehabilitation teams Routine use of exercise physiotherapists Cost of transport |
| Protein energy wasting | Associated with poor prognosis Ensuring adequate nutritional support |
Regular dietetic review and access to nutritional supplements Support in community for shopping, preparing food |
| Multimorbidity | Polypharmacy and risk of adverse reactions Associated with poor prognosis Multiple clinic visits Increased risk of hospitalization |
Involvement of pharmacists to review medications Need to focus goals of care |
The models of care for patients on dialysis were devised at a time when the majority were young and most were eligible for transplantation. As described above, dialysis populations now include a significant proportion of older patients with the median age of those on HD being 67 years in 2014 compared with 64 years in 2000 (MacNeill et al., 2016). Whatever the dialysis modality, they have a high burden of aging-related problems, a high mortality rate, and a high rate of developing the frailty syndrome requiring social support within a few months of starting dialysis, whether on HD or PD (Jassal & Watson, 2009). Such individuals would greatly benefit from a comprehensive geriatric assessment enabling a multiprofessional assessment and access to geriatric and social support services such as falls and memory clinics, physiotherapy, and “hospital at home.” This has been shown in nonrenal populations to identify patients at risk of poor outcome, reduce hospitalization, and is increasingly recommended as being the standard of care in the treatment and decision-making process in older patients with frailty (Ellis et al., 2011).
Renal Elderly Care Integration Project
The demands of being on dialysis make it difficult for older frail patients to access geriatric services, dialysis nurses do not have the necessary skills and are often too busy to coordinate community care, and nephrologists do not incorporate geriatric assessments into routine care. We have therefore set up a project to develop the role of a specialist renal elderly care nurse who can perform a modified geriatric assessment on patients when they are attending the HD unit or PD clinic. This role has been developed by a specialist renal nurse who obtained 2 months of geriatric training at the start of her post by observing geriatric assessments in hospital and community settings. Subsequently, patients were referred to appropriate hospital and community services such as memory clinics, falls clinics, social services, counseling, and physiotherapy. The role has been evaluated assessing patient satisfaction and distress thermometer scores, impact on workload of dialysis nurses, and hospitalization. Patients aged 70 years and over and patients 60 to 70 years who are deemed frail from the PD unit and one of our satellite HD units are referred for assessment.
Patient assessment
A modified geriatric assessment was evolved that could be completed by a single nurse when the patient attended an outpatient clinic or the dialysis unit. This included assessment of dependence on mobility aids, falls, presence of vision or hearing problems, and social support provided by family or other caregivers. Frailty was assessed using the Clinical Frailty Scale (Rockwood et al., 2005). Cognitive dysfunction was measured using the abbreviated mental test score (AMTS) and clock drawing (CLOX) (Royall, Cordes, & Polk, 1998). Patient experience was measured using the Distress Thermometer and the Renal Treatment Satisfaction Score.
Results
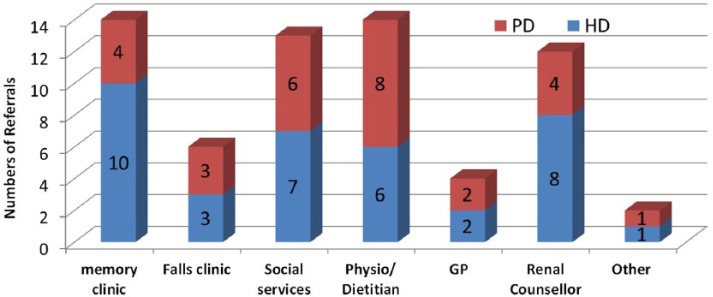
Fifty-eight HD patients (mean age 78.2 ± 5.5 years, 64% male) and 32 PD patients (mean age 76.8 ± 6.1 years, 66% male) have been evaluated. The initial assessment scores are shown in Table 4. Seventy-nine percent HD and 66% PD patients had a Clinical Frailty Score ≥5. Cognitive impairment was common and was found in 27 HD and 14 PD patients. Prior to assessment, only six patients were recognized as having cognitive impairment (four HD and two PD). Executive dysfunction, as measured by CLOX, was more common than memory problems in both HD and PD groups. Moderate to severe distress was found in a quarter of patients. Falls were equally common in both groups with a quarter having had a fall in the last 12 months. Following the initial assessment, 37 HD and 28 PD patients were referred to various community teams and support services as shown in Figure 3. During subsequent follow-up, there were 20 additional referrals to palliative care, Age UK (charity support), dietician, social services, and other support services.
Table 4.
Initial Assessment Scores.
| Assessment | Hemodialysis (58) | Peritoneal dialysis (32) |
|---|---|---|
| % (n) | % (n) | |
| Frailty score = 5 (mild) | 34.4 (20) | 21.8 (7) |
| Frailty score = 6 (moderate) | 41.3 (24) | 43.7 (14) |
| Frailty score = 7 (severe) | 3.4 (2) | 3.1 (1) |
| Abbreviated mental test score ≤ 8 | 20.6 (12) | 12.5 (4) |
| Clock test score <8 | 53.4 (31) | 50.0 (16) |
| Moderate to severe distress (score 5-10) | 27.5 (16) | 25.0 (8) |
| Treatment satisfaction score ≤ 80% | 39.6 (23) | 12.5 (4) |
| Number of patients with falls in last year | 25.8 (15) | 28.1 (9) |
Figure 3.
Referrals to community teams and support services after initial assessment.
Note. PD = peritoneal dialysis; HD = hemodialysis; GP = General Practitioner.
The project started in September 2015. Fifty patients were available for reassessment at 12 months; 14 were not reassessed for various reasons (unwell, transferred to other dialysis units; died before reassessment). Table 5 shows the assessments on the 36 patients (22 HD and 14 PD) who were assessed at baseline and at 12 months. Despite the continuing high prevalence of frailty, with 29 patients (76%) having a clinical frailty score ≥5, there are markedly fewer patients having a high distress thermometer score (6 compared to 13 at baseline) or low treatment satisfaction score (3 compared to 9 at baseline).
Table 5.
Assessment Scores at Baseline and 12 Months.
| Assessments | Initial assessment (22HD and 14PD) | 12 months assessment (22HD and 14PD) |
|---|---|---|
| % (n) | % (n) | |
| Mild frailty score = 5 | 30.5 (11) | 41.6 (15) |
| Moderate frailty score = 6 | 41.6 (15) | 36.1 (13) |
| Severe frailty = 7 | 2.7 (1) | 2.7 (1) |
| Abbreviated mental test score ≤ 8 | 22.2 (8) | 33.3 (12) |
| Clock test score <8 | 58.3 (21) | 61.1 (22) |
| Moderate to severe distress (score 5-10) | 36.1 (13) | 16.6 (6) |
| Treatment satisfaction score ≤ 80% | 25 (9) | 8.3 (3) |
Note. HD = hemodialysis; PD = peritoneal dialysis.
To assess the value of the project, we have also surveyed nursing staff about the value of routine geriatric assessment of older patients. An initial staff survey at the start of the project showed that the majority of nursing staff were involved in providing support services for patients but 64% of HD staff and 40% of PD staff felt that they did not have adequate time to deal with the increasing supportive care needs of older and frail dialysis patients. A repeat survey at 12 months showed a marked decrease in dialysis staff involvement in supportive care needs apart from a continued role in patient falls, reflecting the high incidence, and liaison with individuals’ general practitioner, most commonly regarding medication. Nursing staff commented on the benefits of having the renal elderly care nurse as part of the team with the ability and time to navigate the health and social support network to provide appropriate care with some staff describing the role as a necessity and invaluable in improving patients’ experience and quality of care.
Discussion
Frail and older patients can be challenging for dialysis nurses with limited time or training to address many of the issues that may arise. It could be argued that the management of the needs related to aging should be managed outside the dialysis setting, but realistically this does not happen. General practitioners tend not to get involved and dialysis patients are rarely referred to geriatric services. In any case, comprehensive geriatric assessment, although known to improve outcomes, is time-consuming and requires the input of a several professionals. We therefore decided to develop a more pragmatic approach of developing the role of a renal elderly care nurse who could deliver a modified geriatric assessment on her own.
The high prevalence of frailty, cognitive impairment, and falls found after assessment is well reported in the literature. The higher prevalence of executive dysfunction compared with memory impairment is also well recognized and may have important consequences for the ability of patients to make decisions about their health care (Iyasere, Okai, & Brown, 2017). The high level of poor treatment satisfaction in the HD patients compared with those on PD (39.6% compared with 12.5%) was also found in the Frail Elderly Patient Outcomes on Dialysis study (Iyasere et al., 2016). As discussed by Davison and Jassal (2016), management of these patients should be personalized and focus on improving quality of life rather than on the demands of dialysis targets. The fall in distress thermometer scores and improved treatment satisfaction scores found after 12 months of this project demonstrate the value of this approach.
As well as improving patient supportive care, the introduction of routine geriatric assessment by a specialist renal elderly care nurse has not only resulted in improved patient supportive care and management but has also had a positive impact on staff working patterns as demonstrated by the staff surveys on both the HD and PD units. Patients on the project also reported a positive feedback about the role to staff. The project has resulted in the development of closer working relationship with teams such as geriatric, palliative care, dieticians, counseling, social support, and community teams.
In conclusion, the introduction and development of the role of a renal elderly care nurse performing a routine modified geriatric assessment for older and frail dialysis patients has proved to be beneficial in identifying patients’ needs with referrals to appropriate support services. The integration of geriatrics into renal care has enabled this vulnerable group of renal patients to access much-needed geriatric services with the nurse’s role an integral part of assessing, identifying, planning, and organizing supportive services and care based on needs.
Sociological Perspectives on Renal Transplantation
During the 1950s and 1960s, cases of renal transplant, then an experimental procedure, increased in earnest across the United States and Europe. Renal transplants are of continuing interest to sociologists, not only because the procedure is possible regardless of whether the donor is deceased or still living but also because of the rise in the numbers of older people becoming donors and recipients. Medical sociologists have worked in this field since its inception, with North American sociologists Renee Fox and Judith Swazey (1974) the first to chart its technical development and ethical difficulties, publishing their seminal work “The Courage to Fail.” Significantly, a little less than two decades later their book, Spare Parts: Organ Replacement in American Society (Fox & Swazey, 1992), marked their exit from the field, citing their increasingly troubled and critical reactions to the expansion of human organ replacement.
Expectations of Renal Transplantation
Over time and with repeated reporting of success, many experimental medical interventions are taken into clinical and public consciousness and routinized, such that they not only become one of a number of “standard treatment” possibilities but also part of an increasing range of patient choice. How medicine is practiced and reported by various media in Western society underlies increasing expectations of its capabilities, both on the part of clinicians and patients. Renal transplantation is one such procedure. As time passes, the eligibility criteria for those who can receive the treatment widen; for example, the first HIV positive to HIV positive kidney transplants have recently been conducted. However, decisions around therapy options for patients with end-stage renal failure become more difficult in societies in which understandings of what is “normal” are also shifting. This is not only in what one’s own body can and should appear to be and do, but also in what has become increasingly possible for self and others, and at what life stages these possibilities exist; Jones and Higgs (2010) refer to this as a normalization of diversity. Kaufman, Russ, and Shim (2006, p. 86) illustrate this concept well in the context of a 71-year-old North American patient and their physician deciding whether to consider renal transplantation:
Physician: It depends on how active you are and want to be. Transplant frees you . . . People who want to be active want the added years. It all depends on what you want. It’s a personal choice.
Patient: Now that dialysis has started, I feel I’m in a holding time, a waiting pen. Before I started, I didn’t realize I would think of it this way. I could never stay on it for years. I see transplant as my liberator, the light at the end of the tunnel.
Indeed, much of Western culture has become “death denying,” for example, in believing that most health problems can be overcome or at least ameliorated through medical intervention. Western cultures also deny the fact of aging through the promotion and use of lotions, cosmetic surgery, and advertising that posits youth and vitality as the ideal state, strengthening the notion that the ailing body can be “fixed” or at least maintained in a permanent functioning state.
Currently just over 5,000 people are listed on the United Kingdom’s active kidney-only transplant list, with just under 3,000 kidney-only transplants conducted in the year to April 2016 (NHS Blood and Transplant, 2016). Around one third of these transplants involved a living donor. In 2016, NHS Blood and Transplant announced the 500th altruistic or “nondirected” donor—a living person who donates his or her kidney to a stranger; it predicts around 100 further donations will be made annually. From a clinical perspective, transplanting a living person’s kidney enables control over timing, a lower risk of harm to the recipient, better clinical outcomes, and expands the donor pool to enable more people’s lives to be saved. However, “share your spare,” the tag line of the charity Give a Kidney’s campaign, suggests that human anatomy has an “extra” kidney as a “spare part.” This very recent example of rising expectations of the capabilities of medicine and who is eligible to give and receive a kidney begins another set of normative expectations for renal transplant, and shift again the boundaries of normality.
Kidney Donation as a “Gift”
In the context of blood being donated by unpaid rather than paid donors in the United Kingdom, Titmuss (1971) conceptualized donation of bodily material as “gift giving”; an unconditional gesture that forms part of our civic generosity. However, Mauss (1954) conceptualized the gift relationship as a “gift exchange”: the giver gives, the recipient receives, and in doing so, an obligation is created on the part of the recipient to give something back to the giver. From a sociological perspective, the living kidney transplantation process therefore becomes inherently problematic.
In the context of kidney transplantation, an obligation runs through the whole gift relationship; the relative or friend as donor often has little choice but to at least offer the gift, highlighted in accounts of physicians giving clinical alibis to reluctant donors for their nondonation. The potential recipient cannot easily refuse the offer of the “gift” without denying or risking the relationship that exists between donor and recipient. However, to receive this gift creates an emotional burden. The obligation of reciprocation needs to be met, either by giving back directly to the gift-giver or by giving forward, for example, to a member of a future generation. In the context of renal transplantation, Fox and Swazey (1974, 1992) refer to this dilemma as “the tyranny of the gift”; that is, guilt at what has been accepted and of being forever indebted to the donor.
In the context of giving and receiving a donor organ, transplantation professionals and recipients often refer to the “gift of life.” However, the premise that gift-giving in consumer societies is fundamentally different to commodification (i.e., the kidney as an object that can be bought and sold) has become increasingly meaningless (Shaw, 2010). For instance, many gifts today are associated with a lack of gravity, bought unthinkingly with little effort, with little risk to the giver if the gift is discarded. There is no ethical imperative within the gifting and no debt to repay. It may be necessary therefore to rethink whether the transfer of a kidney from one body to another should be situated within a new, more contemporary discourse.
Sociologists and anthropologists additionally highlight how both the deceased and living donor’s “sacrifice” is hidden within clinical and public discourse, usually only briefly acknowledged in appeals for donors or accounts of successful surgeries (Shaw, 2010). They argue that the “gift of life” discourse plays down this sacrifice, particularly for families who watch loved one on a ventilator looking very much alive. This little-acknowledged sacrifice might help to explain the reluctance of individuals and families to donate, especially when these sacrifices are insufficiently memorialized.
The kidney’s lack of association with any identified emotional quality may be important in its commodification and illegal selling. The Declaration of Istanbul on Organ Trafficking and Transplant Tourism (2008) prohibits organ trafficking, tourism, and commercialism. However, buying and selling kidneys is a growing trade, highlighting increasing global inequalities, the desperate need of those in poverty to provide for their families, and how “sacrifice” is becoming a larger part of the kidney transplantation discourse.
Renal Transplantation for Older People
Due, in part, to the capacity of medical interventions to extend life, Western societies are experiencing profound population aging. In the United Kingdom, those aged 65 years can expect to live an extra 18 to 21 years on average, yet around half this time will be spent with limiting health problems or disability. These demographic and epidemiological shifts ensure that both those waiting for a donor kidney and those who donate are likely to be older; older people are the fastest growing group being assessed for renal transplantation. The latest annual report on kidney transplantation from NHS Blood and Transplant (2016) notes that 30% of registrations on the active waiting list in the year to April 2016 were for those aged 60 years or older, with 6% being 70 years or older.
However, older people currently face more risks from renal transplantation including a longer wait for a kidney from a younger deceased donor, a higher chance of dying on the waiting list, and a shorter life expectancy than younger people posttransplant. In response, older people may be offered “extended” or “expanded” criteria donor organs; those that come from other older people (aged 60 years or older) and those aged 50 to 59 years with vascular comorbidities (Port et al., 2002). These kidneys may not function for as long or as well as those from younger living donors, yet crucially they may become available more quickly. Around 30% of deceased kidney donors in Europe are now expanded criteria donors (Eurotransplant, 2013). Furthermore, the Eurotransplant Senior Program (ESP) for kidney transplantation from donors aged 65 years and over to recipients in the same age category, operational for nearly two decades, is now being evaluated (Eurotransplant, 2015) and if outcomes are positive this area is likely to increase.
In North America, there has been a rise in living donation of kidneys from adult children and grandchildren to their parents and grandparents. In 2003, 1,684 kidneys were given to the over 65s, 515 donated from living donors, 295 of whom were their adult children. Alongside shifting notions of normality is an increasingly blurring boundary between freely given consent and overt or covert pressure to comply. Although many of these older people may not want to consider a living kidney donation, many of Kaufman et al.’s (2006) participants highlight how family pressure might also be put on the patient to accept the offer of a younger relative to donate.
In their North American ethnographic study of older people being assessed and listed for kidney transplantation, Kaufman et al. (2006) explore the decision-making, risk, and uncertainty surrounding expanded donor criteria. For example, the older person needs to decide whether to wait on the list for a “healthy” deceased donor, to accept an expanded criteria donation which may be offered sooner but not last as long, or to consider donation from a living person, who is most likely to be their relative or friend, and all that entails in terms of navigating the “gift exchange.” The older person needs to consider which relatives to approach first, whether to ask a relative explicitly about this or wait for people to offer, or be clear they do not want a living donor. All of these decisions have emotion at their core and are part of what Kaufman et al. (2006) term “a new gerontoethics” (p. 86). In addition, the potential donor has to weigh up the risk to themselves of donating and the emotional implications of doing so, how well and for how long the donated kidney might function, the likelihood of experiencing kidney failure themselves in later life, and their responsibilities to both older and younger family members.
Worryingly, the obligation to give and receive a kidney may also arise from the belief that “that is what families are supposed to do,” in effect where kidneys are taken rather than freely given (Scheper-Hughes, 2007). This creates what Scheper-Hughes terms family bondage rather than family bonds and needs to be considered carefully by clinicians in the context of to what extent informed consent by the donor relative can really be “freely given.” Obligation might also arise for the recipient in the context of the donor conceiving of their kidney as being lent to them; that is that the recipient should live their life in a way in which the donor approves, for example, through a good diet, moral behavior, or more existentially in how they fulfill their life’s purpose.
Box 1. Summary of Role of the Geriatrician in Managing CKD and ESKD in Older People.
1. Identifying and managing risk factors for CKD in older individuals to limit deterioration in renal function through identifying and treating, where possible the more common causes like hypertension, diabetes, and other multisystem diseases.
2. Timely and appropriate referral to specialty care when indicated. For example, advanced stages of CKD (eGFR, 30-45 mL/min/1.73 m2), significant albuminuria (albumin–creatinine ratio 70 mg/mmol), rapid loss of GFR, and refractory hypertension.
3. Assessment of frailty in older patients with CKD. This is essential as an aid in the decision-making process of how best to manage ESRF.
4. Setting up multidisciplinary protocols and regular meetings with nephrology to ensure that older people with CKD have an evidence-based care plan appropriate to the stage and rate of progression of CKD. This will include assessment and appropriate treatment for disease complications such as anemia, bone disease, malnutrition, and psychological issues (e.g., depression).
5. Preparation for RRT or Conservative treatment, ensuring that older people with established CKD receive information on care and understand their options for further management. A management plan should include identification of suitability for RRT, explaining clearly to patient and family the risk entailed and benefits gained. This should also include frail CKD and ESKD patients who are unlikely candidates for RRT but conservative care.
6. Termination of treatment / palliation of ESKD
Conclusion
Renal transplantation creates new and continuing possibilities for kidney donation, recipients’ life enhancement and extension, and the “normality” of pre- and posttransplant status in later life. It continues to throw up new social and ethical challenges, such as the nature of the body and its parts and the position of kidney transfer as a “gift,” moral obligation, commodified transaction, or sacrifice. Increasingly, it suggests kidney transplantation as becoming just one of a number of standard treatment options for older renal patients and their clinicians.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Anand S., Johansen K. L., Kurella Tamura M. (2014). Aging and chronic kidney disease: The impact on physical function and cognition. The Journals of Gerontology: Series A, 69, 315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y., Dalrymple L., Chertow G. M., Kaysen G. A., Johansen K. L. (2012). Frailty, dialysis initiation, and mortality in end-stage renal disease. Archives of Internal Medicine, 172, 1071-1077. doi: 10.1001/archinternmed.2012.3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandna S. M., Silva-Gane D., Marshall C., Warwicker P., Greenwood R. N., Farrington K. (2011). Survival of elderly patients with stage 5 CKD: Comparison of conservative management and renal replacement therapy. Nephrology Dialysis Transplantation, 26, 1608-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. L., Jassal S. V. (2008). Functional dependencies among the elderly on hemodialysis. Kidney International, 73, 1289-1295. [DOI] [PubMed] [Google Scholar]
- Coresh J., Selvin E., Stevens L. A., Manzi J., Kusek J. W., Eggers P., . . . Levey A. S. (2007). Prevalence of chronic kidney disease in the United States. Journal of the American Medical Association, 298, 2038-2047. doi: 10.1001/jama.298.17.2038 [DOI] [PubMed] [Google Scholar]
- Crane M. G., Harris J. J. (1976). Effect of aging on renin activity and aldosterone excretion. The Journal of Laboratory and Clinical Medicine, 87, 947-959. [PubMed] [Google Scholar]
- Dalrymple L. S., Katz R., Rifkin D. E., Siscovick D., Newman A. B., Fried L. F., . . . Shlipak M. G. (2013). Kidney function and prevalent and incident frailty. Clinical Journal of the American Society of Nephrology, 8, 2091-2099. doi: 10.2215/CJN.02870313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison S. N., Jassal S. V. (2016). Supportive care: Integration of patient-centered kidney care to manage symptoms and geriatric syndromes. Clinical Journal of the American Society of Nephrology, 11, 1882-1891. doi: 10.2215/CJN.01050116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis G, Whitehead M. A., Robinson D., O’Neill D., Langhorne P. (2011). Comprehensive geriatric assessment for older adults admitted to hospital: Meta-analysis of randomised controlled trials. British Medical Journal, 343, Article d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M., Hollenberg N. K. (1976). Age as a determinant of renal sodium conservation in normal man. Journal of Laboratory and Clinical Medicine, 87, 411-417. [PubMed] [Google Scholar]
- Eurotransplant. (2013). Annual Report 2013. Retrieved from https://www.eurotransplant.org/cms/mediaobject.php?file=AR20135.pdf
- Eurotransplant. (2015). Annual Report 2015. Retrieved from https://www.eurotransplant.org/cms/mediaobject.php?file=AR_ET_20153.pdf
- Fox R. C., Swazey J. P. (1974). The courage to fail: A social view of organ transplants and dialysis. New Brunswick, NJ: Transaction Publishers. [Google Scholar]
- Fox R. C., Swazey J. P. (1992). Spare parts: Organ replacement in American society. New York, NY: Oxford University Press. [Google Scholar]
- Fried L. P., Tangen C. M., Walston J., Newman A. B., Hirsch C., Gottdiener J. . . . Cardiovascular Health Study Collaborative Research Group. (2001). Frailty in older adults: Evidence for a phenotype. Journals of Gerontology: Series A, Biological Sciences & Medical Sciences, 56, M146-156. [DOI] [PubMed] [Google Scholar]
- Glassock R. J. (2014). Con: Thresholds to define chronic kidney disease should not be age dependent. Nephrology, Dialysis, Transplantation, 29, 774-779; discussion 779-782. doi: 10.1093/ndt/gft306 [DOI] [PubMed] [Google Scholar]
- Glassock R. J., Denic A., Rule A. D. (2017). When kidneys get old: An essay on nephro-geriatrics. Jornal Brasileiro de Nefrologia, 39, 59-64. [DOI] [PubMed] [Google Scholar]
- Grams M. E., Sang Y., Ballew S. H., Gansevoort R. T., Kimm H., Kovesdy C. P., . . . Consortium C. P. (2015). A meta-analysis of the association of estimated GFR, albuminuria, age, race, and sex with acute kidney injury. American Journal of Kidney Diseases, 66, 591-601. doi: 10.1053/j.ajkd.2015.02.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griva K., Stygall J., Hankins M., Davenport A., Harrison M., Newman S. P. (2010). Cognitive impairment and 7-year mortality in dialysis patients. American Journal of Kidney Diseases, 56, 693-703. doi: 10.1053/j.ajkd.2010.07.003 [DOI] [PubMed] [Google Scholar]
- Hallan S. I., Matsushita K., Sang Y., Mahmoodi B. K., Black C., Ishani A. . . . Chronic Kidney Disease Prognosis Consortium. (2012). Age and association of kidney measures with mortality and end-stage renal disease. Journal of the American Medical Association, 308, 2349-2360. doi: 10.1001/jama.2012.16817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmelgarn B. R., Zhang J., Manns B. J., Tonelli M., Larsen E., Ghali W. A., . . . Culleton B. F. (2006). Progression of kidney dysfunction in the community-dwelling elderly. Kidney International, 69, 2155-2161. [DOI] [PubMed] [Google Scholar]
- Iyasere O. U., Brown E. A. (2017). Cognitive function before and after dialysis initiation in adults with chronic kidney disease-a new perspective on an old problem? Kidney International, 91, 784-786. [DOI] [PubMed] [Google Scholar]
- Iyasere O. U., Brown E. A., Johansson L., Huson L., Smee J., Maxwell A. P., . . . Davenport A. (2016). Quality of life and physical function in older patients on dialysis: A comparison of assisted peritoneal dialysis with hemodialysis. Clinical Journal of the American Society of Nephrology, 11, 423-430. doi: 10.2215/CJN.01050115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyasere O. U., Okai D., Brown E. A. (2017). Cognitive function and advanced kidney disease: Longitudinal trends and impact on decision making. Clinical Kidney Journal, 10, 89-94. doi: 10.1093/ckj/sfw128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassal S. V., Chiu E., Hladunewich M. (2009). Loss of independence in patients starting dialysis at 80 years of age or older. New England Journal of Medicine, 361, 1612-1613. [DOI] [PubMed] [Google Scholar]
- Jassal S. V., Watson D. (2009). Dialysis in late life: Benefit or burden. Clinical Journal of the American Society of Nephrology, 4, 2008-2012. [DOI] [PubMed] [Google Scholar]
- Johansen K. L., Chertow G. M., Jin C., Kutner N. G. (2007). Significance of frailty among dialysis patients. Journal of the American Society of Nephrology, 18, 2960-2967. doi: 10.1681/ASN.2007020221 [DOI] [PubMed] [Google Scholar]
- Joly D., Anglicheau D., Alberti C., Nguyen A. T., Touam M., Grünfeld J. P., Jungers P. (2003). Octogenarians reaching end-stage renal disease: Cohort study of decision-making and clinical outcomes. Journal of the American Society of Nephrology, 14, 1012-1021.12660336 [Google Scholar]
- Jones I. R., Higgs P. F. (2010). The natural, the normal and the normative: Contested terrains in ageing and old age. Social Science & Medicine, 71, 1513-1519. [DOI] [PubMed] [Google Scholar]
- Kaufman S. R., Russ A. J., Shim J. K. (2006) Aged bodies and kinship matters: The ethical field of kidney transplant. American Ethnologist, 33, 81-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland J. L., Lye M., Levy D. W., Banerjee A. K. (1983). Patterns of urine flow and electrolyte excretion in healthy elderly people. British Medical Journal (Clinical Research Ed.), 287, 1665-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurella Tamura M., Covinsky K. E., Chertow G. M., Yaffe K., Landefeld C. S., McCulloch C. E. (2009). Functional status of elderly adults before and after initiation of dialysis. New England Journal of Medicine, 361, 1539-1547. doi: 10.1056/nejmoa0904655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurella Tamura M., Vittinghoff E., Hsu C. Y., Tam K., Seliger S. L., Sozio S. . . . CRIC Study Investigators. (2017). Loss of executive function after dialysis initiation in adults with chronic kidney disease. Kidney International, 91, 948-953. doi: 10.1016/j.kint.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurella Tamura M., Yaffe K. (2011). Dementia and cognitive impairment in ESRD: Diagnostic and therapeutic strategies. Kidney International, 79, 14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama N., Mizuno T., Ohshima Y., Yamada K., Ozaki E., Shigeta M., . . . Nakagawa M. (2013). Intracranial deep white matter lesions (DWLs) are associated with chronic kidney disease (CKD) and cognitive impairment: A 5-year follow-up magnetic resonance imaging (MRI) study. Archives of Gerontology and Geriatrics, 56, 55-60. [DOI] [PubMed] [Google Scholar]
- Levey A. S., Stevens L. A., Schmid C. H., Zhang Y. L., Castro A. F., Feldman H. I. . . . Chronic Kidney Disease Epidemiology Collaboration. (2009). A new equation to estimate glomerular filtration rate. Annals of Internal Medicine, 150, 604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman R. D., Tobin J., Shock N. W. (1985). Longitudinal studies on the rate of decline in renal function with age. Journal of the American Geriatrics Society, 33, 278-285. [DOI] [PubMed] [Google Scholar]
- Lønning K., Midtvedt K., Leivestad T., Reisæter A. V., Line P. D., Hartmann A., Heldal K. (2016). Are octogenarians with end-stage renal disease candidates for renal transplantation? Transplantation, 100, 2705-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft F. C., Grim C. E., Willis L. R., Higgins J. T., Weinberger M. H. (1977). Natriuretic response to saline infusion in normotensive and hypertensive man. The role of renin suppression in exaggerated natriuresis. Circulation, 55, 779-784. [DOI] [PubMed] [Google Scholar]
- Macneill S. J., Casula A., Shaw C., Castledine C. (2016). UK Renal Registry 18th Annual Report: Chapter 2 UK renal replacement therapy prevalence in 2014: National and centre-specific analyses. Nephron, 132(Suppl. 1), 41-68. doi: 10.1159/000444816 [DOI] [PubMed] [Google Scholar]
- Matsushita K., Mahmoodi B. K., Woodward M., Emberson J. R., Jafar T. H., Jee S. H. . . . Chronic Kidney Disease Prognosis Consortium. (2012). Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. Journal of the American Medical Association, 307, 1941-1951. doi: 10.1001/jama.2012.3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., van der Velde M., Astor B. C., Woodward M., Levey A. S., de Jong P. E. . . . Chronic Kidney Disease Prognosis Consortium. (2010). Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet, 375, 2073-2081. doi: 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss M. (1954). The gift: Forms and functions of exchange in archaic societies. London, England: Routledge. [Google Scholar]
- McAdams-DeMarco M. A., Tan J., Salter M. L., Gross A., Meoni L. A., Jaar B. G., . . . Sozio S. M. (2015). Frailty and cognitive function in incident hemodialysis patients. Clinical Journal of the American Society of Nephrology, 10, 2181-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moranne O., Couchoud C., Vigneau C. (2012). Characteristics and treatment course of patients older than 75 years, reaching end-stage renal failure in France. The PSPA study. The Journals of Gerontology: Series A, 67, 1394-1399. [DOI] [PubMed] [Google Scholar]
- Murray A. M., Tupper D. E., Knopman D. S., Gilbertson D. T., Pederson S. L., Li S., . . . Kane R. L. (2006). Cognitive impairment in hemodialysis patients is common. Neurology, 67, 216-223. doi: 10.1212/01.wnl.0000225182.15532.40 [DOI] [PubMed] [Google Scholar]
- NHS Blood and Transplant. (2016). Annual report on kidney transplantation (Report for 2015/2016). Retrieved from http://www.odt.nhs.uk/pdf/organ_specific_report_kidney_2016.pdf
- Nickolas T. L., McMahon D. J., Shane E. (2006). Relationship between moderate to severe kidney disease and hip fracture in the United States. Journal of the American Society of Nephrology, 17, 3223-3232. doi: 10.1681/ASN.2005111194 [DOI] [PubMed] [Google Scholar]
- O’Hare A. M., Choi A. I., Bertenthal D., Bacchetti P., Garg A. X., Kaufman J. S., . . . Landefeld C. S. (2007). Age affects outcomes in chronic kidney disease. Journal of the American Society of Nephrology, 18, 2758-2765. doi: 10.1681/ASN.2007040422 [DOI] [PubMed] [Google Scholar]
- Peralta C. A., Shlipak M. G., Judd S., Cushman M., McClellan W., Zakai N. A., . . . Warnock D. (2011). Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. Journal of the American Medical Association, 305, 1545-1552. doi: 10.1001/jama.2011.468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga L. C., Johansen K., Crews D. C., Shahinian V. B., Robinson B. M., Saran R., Powe N. R. (2011). Association of CKD with disability in the United States. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation, 57, 212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F. K., Bragg-Gresham J. L., Metzger R. A., Dykstra D. M., Gillespie B. W., Young E. W., . . . Held P. J. (2002). Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation, 74, 1281-1286. [DOI] [PubMed] [Google Scholar]
- Rockwood K., Song X., macknight C., Bergman H., Hogan D. B., McDowell I., Mitnitski A. (2005). A global clinical measure of fitness and frailty in elderly people. Canadian Medical Association Journal, 173, 489-495. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick P. J., Atkins R. J., Smeeth L., Nitsch D. M., Hubbard R. B., Flectcher A. E., Bulpitt C. J. (2008). Detecting chronic kidney disease in older people; what are the implications? Age and Ageing, 37, 179-186. [DOI] [PubMed] [Google Scholar]
- Ronsberg F., Isles C., Simpson K., Prescott G. (2005). Renal replacement therapy in the over-80s. Age and Ageing, 34, 148-152. [DOI] [PubMed] [Google Scholar]
- Rowe J. W., Andres R., Tobin J. D., Norris A. H., Shock N. W. (1976). The effect of age on creatinine clearance in men: A cross-sectional and longitudinal study. Journal of Gerontology, 31, 155-163. [DOI] [PubMed] [Google Scholar]
- Rowe J. W., Shock N. W., DeFronzo R. A. (1976). The influence of age on the renal response to water deprivation in man. Nephron, 17, 270-278. [DOI] [PubMed] [Google Scholar]
- Royall D. R., Cordes J. A., Polk M. (1998). CLOX: An executive clock drawing task. Journal of Neurology, Neurosurgery & Psychiatry, 64, 588-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule A. D., Amer H., Cornell L. D., Taler S. J., Cosio F. G., Kremers W. K., . . . .Stegall M. D. (2010). The association between age and nephrosclerosis on renal biopsy among healthy adults. Annals of Internal Medicine, 152, 561-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule A. D., Rodeheffer R. J., Larson T. S., Burnett J. C., Cosio F. G., Turner S. T., Jacobsen S. J. (2006). Limitations of estimating glomerular filtration rate from serum creatinine in the general population. Mayo Clinic Proceedings, 81, 1427-1434. doi: 10.4065/81.11.1427 [DOI] [PubMed] [Google Scholar]
- Scheper-Hughes N. (2007). The tyranny of the gift: Sacrificial violence in living donor transplants. American Journal of Transplantation, 7, 507-511. [DOI] [PubMed] [Google Scholar]
- Segall L., Nistor I., Pascual J., Mucsi I., Guirado L., Higgins R., . . . Gavrilovici C. (2016). Criteria for and appropriateness of renal transplantation in elderly patients with end-stage renal disease: A literature review and position statement on behalf of the European Renal Association-European Dialysis and Transplant Association Descartes Working Group and European Renal Best Practice. Transplantation, 100, e55-e65. [DOI] [PubMed] [Google Scholar]
- Seliger S. L., Wendell C. R., Waldstein S. R., Ferrucci L., Zonderman A. B. (2015). Renal function and long-term decline in cognitive function: The Baltimore Longitudinal Study of Aging. American Journal of Nephrology, 41, 305-312. doi: 10.1159/000430922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. (2010). Perceptions of the gift relationship in organ and tissue donation: Views of intensivists and donor and recipient coordinators. Social Science & Medicine, 70, 609-615. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Hoy W. E., Cobb L. (1989). Low incidence of glomerulosclerosis in normal kidneys. Archives of Pathology & Laboratory Medicine, 113, 1253-1255. [PubMed] [Google Scholar]
- Tiffin-Richards F. E., Costa A. S., Holschbach B., Frank R. D., Vassiliadou A., Krüger T., . . . Reetz K. (2014). The Montreal Cognitive Assessment (MoCA)—A sensitive screening instrument for detecting cognitive impairment in chronic hemodialysis patients. PLoS ONE, 9(10), e106700. doi: 10.1371/journal.pone.0106700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titmuss R. M. (1971). The gift relationship: From human blood to social policy. New York, NY: Pantheon Books. [Google Scholar]
- van der Velde M., Matsushita K., Coresh J., Astor B. C., Woodward M., Levey A., . . . Consortium C. K. D. P. (2011). Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney International, 79, 1341-1352. doi: 10.1038/ki.2010.536 [DOI] [PubMed] [Google Scholar]
- Weidmann P., De Myttenaere-Bursztein S., Maxwell M. H., de Lima J. (1975). Effect of aging on plasma renin and aldosterone in normal man. Kidney International, 8, 325-333. [DOI] [PubMed] [Google Scholar]
- Wesson L. G. (1969). Physiology of the human kidney. New York, NY: Grune & Stratton. [Google Scholar]
- Wetzels J. F., Kiemeney L. A., Swinkels D. W., Willems H. L., den Heijer M. (2007). Age- and gender-specific reference values of estimated GFR in Caucasians: The Nijmegen Biomedical Study. Kidney International, 72, 632-637. doi: 10.1038/sj.ki.5002374 [DOI] [PubMed] [Google Scholar]
- Wolfgram D., Szabo A., Murray A. M., Whittle J. (2015). Risk of dementia in peritoneal dialysis patients compared with hemodialysis patients. Peritoneal Dialysis International, 35, 189-198. doi: 10.3747/pdi.2014.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. J., Rakheja D., Yu X., Saxena R., Vaziri N. D., Silva F. G. (2008). The aging kidney. Kidney International, 74, 710-720. [DOI] [PubMed] [Google Scholar]