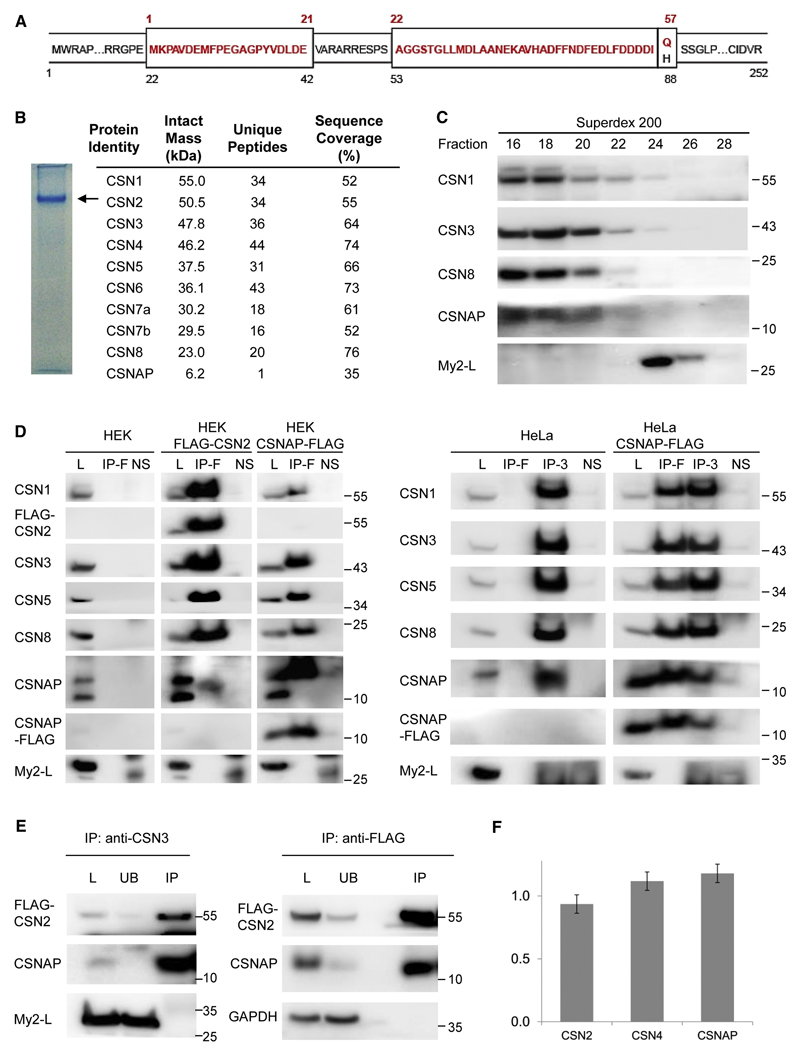
Figure 2. The short isoform of the Myeov2 gene, CSNAP, interacts with the CSN complex.
(A) Schematic representation of the two alternatively spliced products of the Myeov2 gene. Amino acid residues colored in red represent the CSNAP sequence (57 amino acids). The long Myeov2 protein (252 amino acids, My2-L) contains the entire sequence of the short transcript except for glutamine 57, as well as additional sequence stretches in internal and end regions (shown in black). (B) Native PAGE separation (6%) of the purified CSN complex. The position of the complex is denoted by an arrow; the absence of additional bands in the gel indicates the high integrity of the complex. Proteins extracted from the labeled band were subjected to proteomic LC-MS/MS analysis. Identified proteins with sequence coverage ≥ 30% are listed. (C) HEK293T FLAG-CSN2 cell extract was subjected to Superdex 200 gel-filtration chromatography. Fractions were collected and analyzed by SDS-PAGE and immunoblotting. CSNAP co-fractionated with the CSN complex, unlike Myeov2-L, that eluted in lower molecular weight fractions. (D) Whole-cell lysates (L) from HEK293T cells stably expressing FLAG-CSN2, or HEK293 cells transiently expressing CSNAP-FLAG (left panel), as well as lysates from HeLa cells transiently expressing CSNAP-FLAG (right panel), were subjected to immunoprecipitation (IP) using FLAG affinity gel (IP-F) or anti-CSN3 antibody (IP-3). Pulldowns were analyzed by Western blots, using antibodies against CSN subunits, CSNAP, and FLAG. The results show that CSNAP, but not the long version of Myeov2, co-immunoprecipitate with the CSN complex. As controls, WT HEK293 and HeLa lysates were used. To rule out non-specific interactions, lysates were also incubated with Protein G Sepharose, without the addition of the primary antibodies (NS). (E) CSN was co-immunoprecipitated from HEK293 stably expressing FLAG-CSN2, using an anti-CSN3 antibody or anti-FLAG resin. The whole-cell lysates (L) bound (IP) and unbound (UB) fractions were analyzed by Western blots using antibodies against CSNAP and FLAG. GAPDH and My2-L were used as negative controls. The depletion of CSN2 and CSNAP from the unbound fraction not only suggests that the majority of CSN complexes are bound to CSNAP, but also that this small protein is not part of another protein complex. In all Western blots, molecular weights are indicated in kDa units. All experiments were repeated at least 3 times. (F) CSN was subjected to targeted proteomic analysis by selective reaction monitoring (SRM) mass spectrometry and stable, isotopically labeled peptide standards. Absolute quantification was done by referencing the native peptide intensities to the heavy labeled standards, and then normalizing against a representative peptide. Results indicate that CSN subunits and CSNAP are present in equimolar amounts. Data shown are the result of three biological replicates, with two technical replicates each. Error bars indicate the standard deviations of all six measurements.