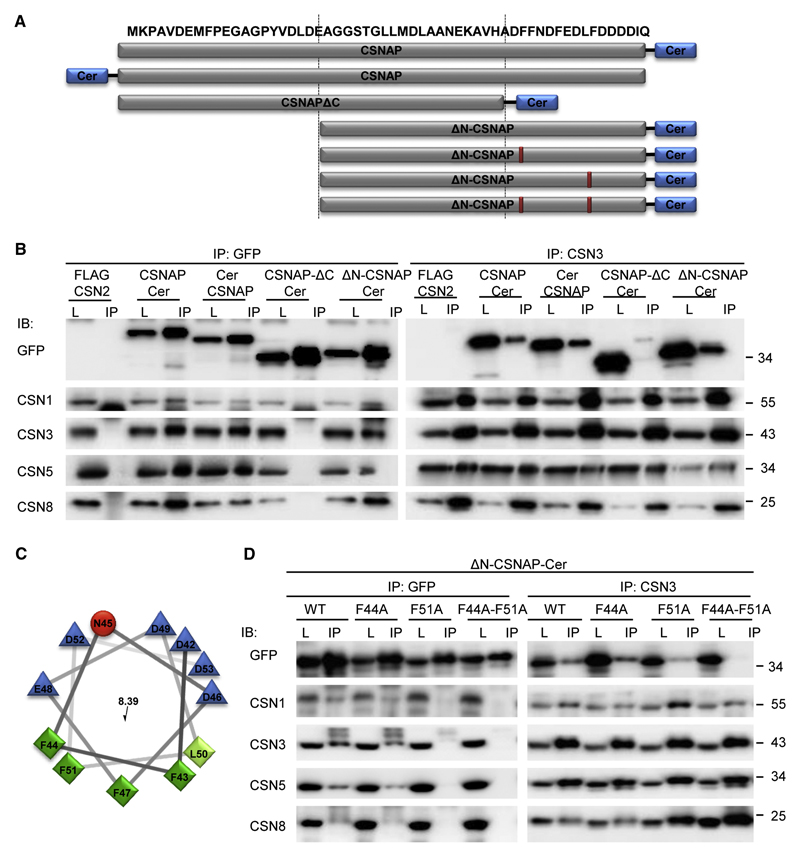
Figure 3. The F/D-rich C-terminal domain of CSNAP, specifically Phe44 and Phe51, are involved in its interaction with the CSN complex.
(A) Schematic representation of the different CSNAP constructs used in this experiment. (B) Cellular proteins extracted from the different fluorescently tagged HEK293 cell lines were immunoprecipitated, using anti-GFP and anti-CSN3 antibodies. As control, lysate from HEK293 cells stably expressing FLAG-CSN2 was used. Lysates (L) were run side-by-side with their corresponding immunoprecipitated proteins (IP), and visualized using various antibodies, as indicated (IB). Results show that CSNAP-ΔC-Cer did not interact with the CSN, indicating that the C-terminal domain is responsible for its interaction with the complex. (C) Helical wheel representation of the CSNAP C-terminal region. Hydrophilic and hydrophobic residues are colored blue and green, respectively. Distribution of the residues on either side of the helix suggests amphipathic properties for this structure. (D) Lysates (L) from HEK293 cells expressing ΔN-CSNAP-Cer (WT) and its mutational variants, consisting of single (F44A and F51A) and double (F44A-F51A) amino acid substitutions, were immunoprecipitated by either anti-GFP or anti-CSN3 (IP). Pulldowns were analyzed by Western blot, using antibodies against GFP and CSN subunits. Findings show that while Phe44 displacement yielded results similar to those of the WT construct, the F51A mutant extensively weakened the interaction of ΔN-CSNAP with the CSN. The most pronounced effect was observed for the F44A-F51A double mutant, which entirely abolished the ΔN-CSNAP/CSN interaction.