Abstract
Background
Clinical trials have established the average benefit of cardiac resynchronization therapy (CRT), but estimating benefit for individual patients remains difficult because of the heterogeneity in treatment response. Accordingly, we created a multivariable model to predict changes in quality of life (QoL) with and without CRT.
Methods and Results
Patient-level data from 5 randomized trials comparing CRT with no CRT were used to create a prediction model of change in QoL at 3 months using a partial proportional odds model for no change, small, moderate and large improvement or deterioration of any magnitude. The c-statistics for not worsening or obtaining at least a small, moderate, and large improvement were calculated. Among the 3614 patients, regardless of assigned treatment, 33.3% had a deterioration in QoL, 9.2% had no change, 9.2% had a small improvement, 13.5% had a moderate improvement, and the remaining 34.9% had a large improvement. Patients undergoing CRT were less likely to have a decrement in their QoL (28.2% vs. 38.9%, p<0.001) and more likely to have a large QoL improvement (38.7% vs. 30.6%, p<0.001). A partial proportional odds model identified baseline QoL, age, and an interaction of CRT with QRS duration as predictors of QoL benefits 3 months after randomization. C-statistics of 0.65 for not worsening, 0.68 for at least a small improvement, 0.69 for at least a moderate improvement, and 0.73 for predicting a large improvement were observed.
Conclusions
There is marked heterogeneity of treatment benefit of CRT that can be predicted based upon
Keywords: Cardiac Resynchronization Therapy, Quality of Life, Heterogeneity of treatment
Journal Subject Code: [37] CV, Cardiac Resynchronization therapy, cardiomyopathy, [110] Congestive (Heart Failure), Quality of Life
For patients with advanced systolic heart failure and a widened QRS, guidelines recommend several therapies, including cardiac resynchronization therapy (CRT), to improve survival and quality of life (QoL).1, 2 It is inherently difficult, however, to apply guidelines to individual patients whose benefits from treatment may differ substantially from the ‘average’ benefit described in clinical trials. This issue is of particular concern for CRT, which requires a relatively complex, expensive, invasive procedure compared to a pharmacological therapy that can easily be given for a trial period and easily withdrawn if it causes problems. Also, the response to CRT is heterogeneous, some patients improve while others worsen. In general, patients are 8–10% more likely to experience a favorable improvement in disease specific quality of life with vs. without CRT.3–6
Previous efforts to create risk models to personalize the estimated benefits of CRT have focused on survival.7 Importantly, such models do not inform patients and their providers of the likely benefits of CRT on the patient’s QoL.3 Given the importance of QoL to patients,8 formally modeling the heterogeneity of treatment benefit for QoL outcomes can assist physicians in patient selection, enable more accurate discussions of the risks and benefits of treatment and support shared medical decision-making.9–11 To address the need for a tool to individualize treatment based upon anticipated QoL benefits for individual patients, we used patient-level data from 5 randomized trials of CRT to develop a multivariable risk prediction model of QoL benefit after CRT.
METHODS
Data Source
Patient-level data were pooled from 5 randomized controlled trials of CRT therapy and included 4317 patients. Details of the CARE-HF, MIRACLE, MIRACLE-ICD, REVERSE, and RAFT studies have been described previously.3, 4, 12–14 Each trial was approved by an institutional review board and informed consent was obtained. The pooled data set was completely deidentified and considered non-human subjects research. While there were differences among the trials, all included adult patients with a diagnosis of chronic heart failure, left ventricular dysfunction, and a wide QRS complex. All trials had at least 6 months of follow-up and collected disease-specific health status data at baseline and follow-up. Given the paucity of data on patients with New York Heart Association (NYHA) class I, we restricted the analyses to those with NYHA II-IV.
Health Status Measures
Disease-specific health status was assessed in the trials using the Minnesota living with heart failure (MLWHF) and Kansas City Cardiomyopathy questionnaire (KCCQ).15, 16 The MLWHF was collected in all 5 trials, while the KCCQ were collected only in REVERSE. The MLWHF contains 21 questions and has a range in overall scores of 0–105 points, with lower scores indicating better QoL. The KCCQ is a 12- or 23-item instrument that ranges in overall scores from 0–100 points, where higher scores indicate better health status.17 Both instruments have been shown to be reliable, responsive, and valid measures of patients’ heart failure symptoms, functional status, and disease-specific QoL. However, the data for interpreting what constitutes clinically important changes in overall score has been more clearly defined for the KCCQ,18 where a 5-, 10-, and 20-point change in the KCCQ Overall Summary score corresponds to a small, moderate, and large clinical change in patients’ health status.8, 18, 19
Selecting the time for modeling health status benefits
Given the variability in the timing of QoL collection amongst the trials, we needed to define a suitable time for modeling follow-up health status. The most QoL data were available at 3 months (Appendix Table S1). However, to ensure that 3 months was a sufficient length of time for the QoL benefits of CRT to be attained, we evaluated the change in QoL from baseline to 3, 6 and 12 months. For the MLWHF, across all patients, there was a mean improvement of −12.4 from baseline to 3 months. At 6 months, the mean MLWHF change from baseline was −12.7, with no significant difference between the changes from baseline at month 3 and month 6, (p-value 0.10); Appendix Figure S1). We therefore selected the 3-month health status assessment to model the heterogeneity of treatment benefit from CRT. To evaluate the models performance on longer-term outcomes, a sensitivity analysis evaluating calibration and discrimination was conducted on patients with 12 month QoL data available.
Defining Clinically Important Changes in QoL
To improve the clinical interpretability of the models and support their use in patient selection and shared decision-making, we sought to model clinically important categories of change, rather than modeling the MLWHF as a continuous score. Given that there are much clearer thresholds to interpret the magnitude of change that is clinically important with the KCCQ, we modeled the improvement in the MLWHF associated with small, moderate or large improvements in KCCQ scores.18, 19 To perform this analysis, we used data from REVERSE, which simultaneously collected both the MLWHF and KCCQ. Changes in KCCQ from baseline to 3 months suggested that a small clinical improvement in the MLWHF questionnaire was −6.67 points; a moderate improvement was −10.41 points; and a large clinical improvement was −17.90 points (Supplemental S1).
Statistical Analyses
As particular patients may find different levels of change in QoL clinically relevant, we used a cumulative logit model to estimate the heterogeneity of treatment benefit with CRT. This cumulative logit model can be thought of as an extension of the logistic regression model that applies to dichotomous dependent variables, allowing for more than two (ordered) response categories. It uses cumulative probability up to a possible threshold, thereby making the whole range of ordinal categories binary at that particular threshold. In this study, we used 4 cutoff points for MLWHF change from baseline at 3 months (−2.92 reflecting deterioration, <−6.67 for a small QoL improvement, <−10.41 for a moderate QoL improvement, and −17.9 for a large QoL improvement). The cumulative logit model thus enables the estimation of the probabilities that a patient will be worse (>−2.92 points), unchanged (−2.92 to −6.67 points), slightly (−6.67 to −10.4 points), moderately (−10.4 to −17.9 points) or substantially better (<−17.9 points) at 3 months.
Candidate variables were selected a priori on the basis of published literature and clinical experience and included: age, sex, angiotensin converting enzyme inhibitors (ACE-I) or angiotensin II receptor blockers (ARB), beta-blockers, left ventricular ejection fraction (LVEF), left ventricular end diastolic dimension, diabetes, QRS duration, left bundle branch block (LBBB), MLWHF at baseline, CRT, atrial rhythm, and ischemic etiology of cardiomyopathy. Spironolactone use and NYHA class at baseline were both highly collinear with baseline QoL and thus omitted as candidate variables. Interactions between CRT and other variables were examined and retained if statistically significant. We used stepwise variable selection to select the final variables for the model. The assumption of linearity was assessed for each continuous variable with the use of restricted cubic splines. The proportional odds assumptions were tested for all variables included in the final model, and assumptions held for all variables except baseline QoL, requiring separate intercepts for each category of 3-month QoL change. To take into clustering of patients by study, study was included a random effect.
Discrimination (c statistic) was calculated for each binary cumulative outcome at each threshold of clinical change. In sensitivity analysis, calibration plots were created by comparing observed versus predicted probability by decile of no change, small improvement, moderate improvement, and large improvements in QoL. Statistical analyses were conducted with SAS version 9.3 (SAS Institute, Cary, NC) and R version 2.7.2.
RESULTS
Study Population
Of the 4317 patients from the 5 trials, 98 patients were excluded as they died prior to the 3-month QoL assessment (censored at end of randomization period). Patients not surviving to 3-months were older, with a worse QoL and generally sicker at baseline (Supplemental table S2). We also excluded patients without baseline (n=117) or 3-month QoL data (n= 388), and excluded the patients with NYHA class 1 (n= 100), leaving 3614 patients for this analysis, of whom 1890 were assigned to CRT. Patients excluded for missing QoL data had better baseline QoL and were more likely to be on a beta blocker, however, other baseline characteristics were similar to the analytic cohort (Supplemental table S3). Mean age of the cohort was 65±10 years, 78% were men, 30% had diabetes, and 58% had an ischemic cardiomyopathy. Mean LVEF was 24±6%, mean QRS duration was 162±24 ms, and 76% had a left bundle branch block (LBBB). Mean MLWHF at baseline was 42.4±23.4. Baseline characteristics between CRT and controls were generally well balanced with the exception of more patients in the CRT cohort being NYHA II (52.2% vs. 48.3%, p = 0.017) and on beta blockers (80.3% vs. 76.7%, p = 0.008) (Supplemental table S4).
Change in QoL and CRT Effect
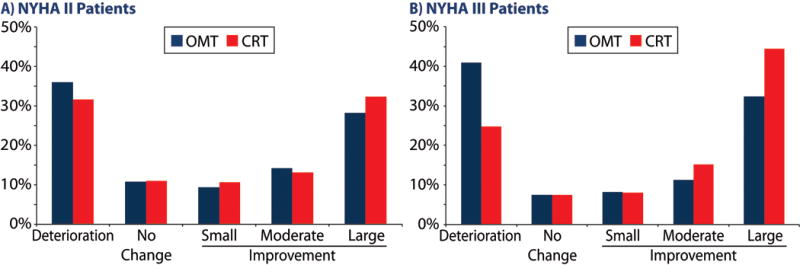
Among the 3614 patients, regardless of assigned treatment, 33.3% had deterioration in QoL, 9.2% had no change, 9.2% had a small improvement, 13.5% had a moderate improvement, and the remaining 34.9% had a large improvement. The baseline characteristics of patients, stratified by change in QoL, are presented in Table 1. From baseline to 3 months, the MLWHF score improved, on average, by −14.0 ± 20.6 with CRT vs. −10.3 ± 20.8 with optimal medical therapy (OMT) for a mean difference of 3.7 points (p<0.001), which is less than the estimated minimally important difference of −6.67 points. However, there was considerable heterogeneity in response (Figure 1). For patients in NYHA III or IV at baseline (n=1795), the mean difference between CRT and OMT was −6.3 (p < 0.0001) points but for those in NYHA II (n=1819), it was only −1.5 points (p = 0.10). In a responder analysis, which categorizes patients’ changes in QoL into worse (>−2.92 points), unchanged (−2.92 to 6.67 points), slightly (−6.67 to −10.4 points), moderately (−10.4 to −17.9 points) or substantially better (<−17.9 points), patients undergoing CRT were less likely to have a decrement in their QoL (28.2% vs. 38.9%, p<0.001) and more likely to have a large QoL improvement (38.7% vs. 30.6%, p<0.001; Supplemental Table 5). These differences were more marked in patients who were in NYHA III or IV at baseline compared to those in NYHA II, respectively (Figure 2a and 2b).
Table 1.
Baseline Characteristics by Change in Quality of Life at 3 Months
| Deterioration n=1203 |
No Change n=332 |
Small Improvement n=331 |
Moderate Improvement n=488 |
Large Improvement n=1260 |
p-value | |
|---|---|---|---|---|---|---|
| Age (y) | 65.5 ± 10.3 | 66.6 ± 9.8 | 65.6 ± 10.2 | 64.1 ± 10.8 | 64.7 ± 10.1 | 0.004 |
| Male | 977 (81.2%) | 273 (82.2%) | 262 (79.2%) | 378 (77.5%) | 937 (74.4%) | <0.001 |
| QRS width (ms) | 161 ± 24 | 161 ± 23 | 163 ± 24 | 164 ± 24 | 163 ± 24 | 0.061 |
| Left bundle branch block | 73.7% | 76.1% | 78.2% | 76.5% | 76.6% | 0.349 |
| Cardiac resynchronization therapy | 44.3% | 53.6% | 54.4% | 54.7% | 58.1% | <0.001 |
| Implantable defibrillator | 65.1% | 67.5% | 66.2% | 64.1% | 65.9% | 0.878 |
| NYHA Class | ||||||
| II | 620 (51.5%) | 201 (60.5%) | 186 (56.2%) | 251 (51.4%) | 561 (44.5%) | < 0.001 |
| III | 554 (46.1%) | 125 (37.7%) | 137 (41.4%) | 222 (45.5%) | 647 (51.3%) | |
| IV | 29 (2.4%) | 6 (1.8%) | 8 (2.4%) | 15 (3.1%) | 52 (4.1%) | |
| Left ventricular ejection fraction (%) | 24.2 ± 6.1 | 24.5 ± 6.3 | 24.2 ± 6.6 | 24.1 ± 6.1 | 23.6 ± 6.1 | 0.024 |
| Systolic blood pressure (mmHg) | 118.4 ± 18.1 | 119.3 ± 18.5 | 119.5 ± 17.5 | 117.8 ± 17.6 | 117.1 ± 17.7 | 0.083 |
| Ischemic cardiomyopathy | 703 (58.4%) | 204 (61.4%) | 187 (56.5%) | 257 (52.7%) | 749 (59.4%) | 0.066 |
| Diabetes mellitus | 30.8% | 30.0% | 28.6% | 28.4% | 30.1% | 0.901 |
| MLWHF at baseline | 31.6 ± 21.1 | 31.8 ± 22.6 | 37.5 ± 22.1 | 42.6 ± 21.7 | 56.6 ± 18.7 | <0.001 |
| ACE-I/ARB | 1152 (95.8%) | 317 (95.5%) | 316 (95.5%) | 467 (95.7%) | 1193 (94.7%) | 0.760 |
| Beta blocker | 935 (77.7%) | 285 (85.8%) | 261 (78.9%) | 384 (78.7%) | 976 (77.5%) | 0.018 |
| Spironolactone | 43.9% | 40.6% | 38.7% | 48.3% | 43.9% | 0.080 |
Values are shown as absolute numbers (percentages), mean ± SD. NYHA, New York Heart Association; MLWHF, Minnesota Living with Heart Failure; ACE-I, angiotensin converting enzyme-inhibitor; ARB, angiotensin II receptor blocker.
Figure 1. Individual patient’s baseline (X axis), and 3 month (Y axis) Minnesota Living With Heart Failure overall score after being assigned to receive cardiac resynchronization therapy (a) and medical therapy (b).
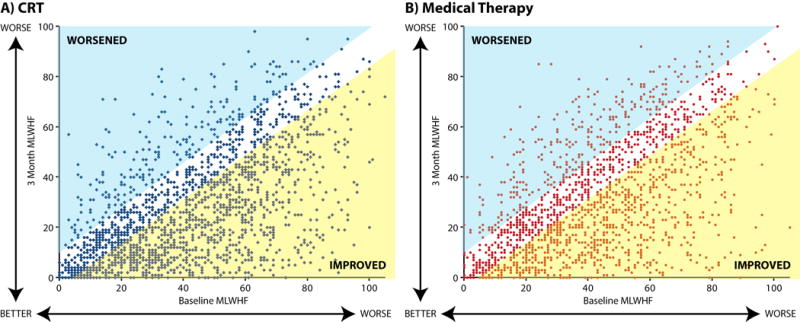
Yellow shaded area represents patients with significant improvement in quality of life. Blue shaded area represents patients with significant worsening in quality of life.
Figure 2. Responder analysis of quality of life change from baseline to 3 months by NYHA II patients (a) and NYHA III/IV patients (b).
Predictors of QoL Improvement
The partial proportional odds model identified baseline QoL, age, and an interaction of CRT with QRS duration as significant predictors of improvement in QoL at 3 months. (Figure 3). Patients with wider QRS duration had the greatest benefit from CRT. Worse baseline QoL and older age were associated with more improvement at 3 months, regardless of treatment. Baseline QoL had different risks for different magnitudes of clinical change, but overall the lower the baseline health status, the greater likelihood of a large improvement in QoL over 3 months' follow-up, regardless of CRT. The discrimination (c-statistics) of the partial proportional odds model for not worse, at least small improvement, at least moderate improvement, and large improvement were 0.65, 0.68, 0.69, and 0.73, respectively. A sensitivity analysis of the models performance among subjects with 12-month QoL data available was conducted. At 12-months the model had better discrimination with c-statistics of 0.71, 0.74, 0.76 and 0.79 for not worse, at least small improvement, at least moderate improvement, and large improvement, respectively, and demonstrated excellent calibration (see Figures S2–S5)
Figure 3. Forest plot of odds ratios from partial proportion odds model (higher scores in MLWHF indicate a worse quality of life).
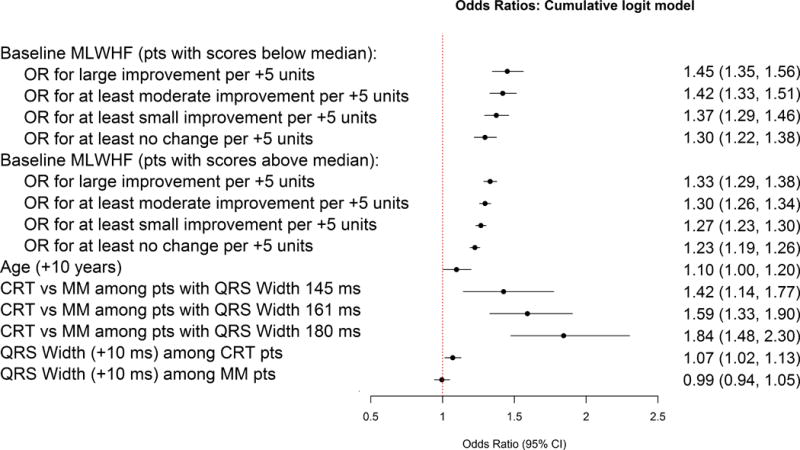
MLWHF = Minnesota Living With Heart Failure; CRT = cardiac resynchronization therapy; MM = medical therapy.
Figure 4 demonstrates a potentially actionable output format for these models when used at the bedside for clinical decision making. In this example, a 72-year old with a baseline MLWHF of 52 and QRS duration of 175 ms would have a 54% probability of a large QOL improvement with CRT as compared to 33% without CRT (NNT = 5). Conversely a 35-year-old with a baseline MLWHF of 40 and QRS duration of 130 would have only a 36% probability of a large improvement with CRT vs. 31% without CRT (NNT = 20).
Figure 4. Example model output for patient shared decision making.
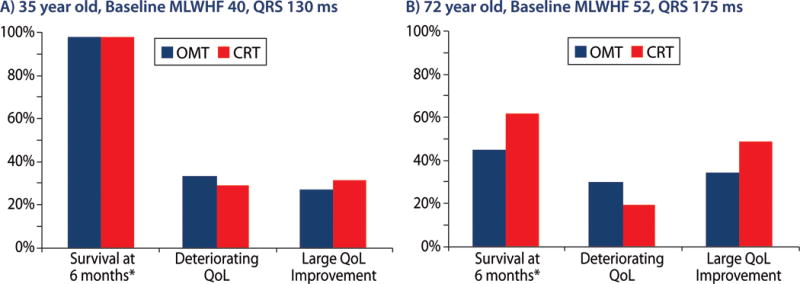
Predicted risk estimates are depicted with bars; lines represent 95% confidence intervals. Estimates of mortality taken from Cleland et al.7 NICM indicates non-ischemic cardiomyopathy; LBBB indicates left bundle branch block, RBBB indicates right bundle branch block.
DISCUSSION
Despite the undoubted benefits of CRT, there are concerns that it has been underused in clinical practice, with only about a third of eligible patients being treated.20, 21 More appropriate use of CRT has been estimated to potentially save more than 8,000 lives per year in the USA alone.21 A potential reason for the underuse of CRT is the difficulty in weighing the risks and benefits the procedure for a specific patient. While a substantial amount of work has been done to try to understand which patients will respond to CRT, benefit has been measured in terms of echocardiographic response or a reduction in morbid or fatal events, despite the fact that patients may care more about the quality of their lives than its quantity.22 To extend prior reports of the average QoL benefits of CRT in a study population, we developed a method for personalizing the estimated likelihood of QoL improvement with CRT. Such a model could support more evidence-based, patient-centered care by helping patients and their providers understand the benefits that they might expect from CRT, especially when coupled with a model estimating the CRT benefits on survival.7
Our findings extend prior investigations of the QoL benefits of CRT, which have shown increasing survival with longer QRS duration and in those with more severe heart failure but have not been able to clearly define patient characteristics associated with improved QoL.4, 7, 23, 24 Our analysis found that after adjusting for QRS duration, QRS morphology does not predict the response to CRT, consistent with two prior patient level analyses14, 25 but not another.26 It is notable that the majority of patients (76%) in our analysis had LBBB, and it is possible that those with wide right bundle branch block (RBBB) had some underlying left sided dyssynchrony, so extrapolation to those with pure RBBB may be limited. Also contrary to some previous analyses27, 28, gender was not predictive of QoL improvement in the final multivariable model, despite women deriving more benefit in unadjusted analyses. We hypothesize that this is due to the worse QoL scores in women at the time of treatment and that once baseline QoL was accounted for gender was no longer independently associated with CRT benefit. Moreover, we found that baseline QoL was very important, where worse baseline health status was associated with a greater likelihood of improvement. Older patients obtained greater benefit in QoL, despite prior studies showing less use of CRT in older patients.29 Using a coarser assessment of QoL than the MLQHF questionnaire, the NYHA classification, we found more benefit in those with NYHA III/IV as compared to those with NYHA II.
Clinical Implications
Our data provide an opportunity to estimate the QoL benefits for an individual patient and to use this information, coupled with mortality and peri-procedural risks, to engage patients in shared decision-making about CRT.7, 30 While, our tool only assesses the initial benefits of CRT it is reassuring that the model continued to perform well at 12 month post implant. Further work will be needed to define strategies for optimizing CRT in order to maximize QoL improvement, and to define the longer-term QoL benefits of treatment.
Potential Limitations
Our decision to include a patient reported outcome (PRO) into the model as opposed to more commonly available NYHA class may hinder implementation. Our rationale to include MLHWF (or PROs in general) as opposed to NYHA class is that the later is a very crude measurement and subject to ‘gaming’. There have been several articles explicitly demonstrating low inter-rater reliability of the NYHA with a concordance between 2 different cardiologists’ assessments of the same patient of about 0.54.31–33 In contrast, the intra-class correlation of health status questionnaires, such as the KCCQ, are quite high with a an estimate of ~0.92 in stable patients.34 Further underscoring the value of collecting PRO data is the accuracy of the model when a more detailed, reproducible assessment of health status is used. For these reasons, we believe that the improved accuracy of the models is greater and justifies the added burden of collecting patients’ health status with a questionnaire.
Our findings should be considered in the context of several potential limitations. QoL change from baseline to 3 months was used as our endpoint since the largest amount of follow up QoL data was available at 3 months and most of the QoL benefit occurred within this timeframe. In sensitivity analysis the model performed well at 12 months, however, there was not an opportunity to explore longer-term outcomes that may have been associated with greater QoL benefits, which might be particularly relevant in patients with NYHA II. This will require future research with longer-term outcomes, which may be important given the evidence of continuing positive remodeling of the left ventricle up to 18 months after CRT implantation.35, 36 Second, as there are not well-developed thresholds of clinical change for the MLHFQ, we had to model these thresholds based upon the clinically-important thresholds of change defined by the KCCQ. We used linear regression from patients with simultaneous MLHFQ and KCCQ in REVERSE to estimate clinical meaningful thresholds in MLFHQ because much more work has been conducted to define clinically important thresholds of change in the KCCQ than the MLHFQ. While we conducted sensitivity analyses and found consistent results, this methodology hasn’t been validated in other datasets and REVERSE included healthier patients than many CRT trials. Future efforts to better map the MHLFQ to the KCCQ could validate our estimates and might change, presumably modestly, the results of our model by using a different threshold of clinically significant change. Third, we used older CRT studies, which could impact generalizability of our findings to current practice, as our model may underestimate the benefits of CRT with newer, more advanced devices (quadripolar leads, multi-point pacing, adaptive CRT, etc.). While these evolutions in technology may underestimate the benefits of CRT, it is also possible that evolving medical therapies may have improved the health status of patients not treated with CRT. Thus, these models should be considered an initial step in an evolving effort to validate and improve patient-specific outcome estimates. Fourth, the controls groups of the pooled data were not identical, while MIRACLE, MIRACLE-ICD, REVERSE, and RAFT had devices implanted, but turned off, CARE had only medical therapy. It is thus possible that the greater QoL benefit in CARE may have reflected a placebo effect, further underscoring the value of including all trials in our analyses. Fifth, while independent patient data was available, certain lab values and information about peripheral artery disease, lung disease, and ICD shocks (appropriate and inappropriate) were not available to be included in the model. Finally, our model has not been validated in other RCTs or in registries and external validation of these models should be pursued.
Conclusions
We identified substantial variability in the benefits of CRT on QoL, which could be modeled using only 3 variables, age, baseline health status and QRS duration. This model may contribute to the infrastructure for personalizing the benefits of CRT for those being considered for this intervention. Future studies should examine whether the prospective use of this and models that predict procedural risks and long-term morbidity and mortality can improve patients’ participation in shared decision-making, target the use of CRT to patients most likely to benefit, and whether this approach to precision medicine can enhance the ability of this important technology to improve the outcomes of patients with heart failure.
Supplementary Material
What is new?
The results of clinical trials average the benefit of treatment across a population, but the expected benefits for individual patients can vary substantially.
While Cardiac Resynchronization Therapy (CRT) has a small average benefit on patients’ health status (their symptoms, function and quality of life), some patients benefit a lot and others don’t.
Using 5 randomized clinical trials, we built a model to predict the health status benefits of CRT for individual patients using only 3 variables; age, QRS width and baseline health status.
What are the Clinical Implications?
By using this new model, clinicians can calculate whether patients are likely to have a large, moderate or small health status benefit from CRT, or whether they are unlikely to feel worse 3 or 12 months after treatment.
The results of this model cannot only inform clinicians, but can also be shared with patients as a foundation for shared medical decision-making.
Future studies to define the benefit of this tool in patient engagement, clinical outcomes and as a component of disease management should be tested.
Acknowledgments
Sources of Funding: This project has been funded in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201100025C. MEN is supported by a T32 grant from the NHLBI (T32HL110837). SVA: supported by a Career Development Grant Award from the NHLBI (K23 HL116799).
Footnotes
Disclosures: M.E.N: None. J.G.C.: other research support (modest)—Biotronik; consultant/advisory board (modest)—Biotronik, St Jude Medical. W.T.A.: consultant/advisory board (significant)—Biotronik, Medtronic St Jude Medical. C.L.: research grant (modest)—Medtronic; other research support (modest)—Medtronic; honoraria (modest)—Biotronik, St Jude Medical; consultant/advisory board (modest)—St Jude Medical. M.R.G.: research grant (significant)—Medtronic, St Jude Medical; speakers bureau (modest)—Biotronik; consultant/advisory board (modest)— Sorin; consultant/advisory board (significant)—Boston Scientic, Medtronic, St Jude Medical. J.B.Y.: consultant/advisory board (modest)— Medtronic. J.C.D.: research grant (modest)—Medtronic; consultant/advisory board (modest)—Medtronic, St Jude Medical. L.S.: employment (significant)—Medtronic. G.A.W.: research grant (significant)—St Jude Medical; consultant/advisory board (significant)—Medtronic. A.S.L.T.: research grant (significant)—St. Jude Medical; consultant/advisory board (significant)—Medtronic
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology F. American Heart Association Task Force on Practice G 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE, Guidelines ESCCfP. Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, Kirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bansch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA) Eur Heart J. 2013;34:2281–329. doi: 10.1093/eurheartj/eht150. [DOI] [PubMed] [Google Scholar]
- 3.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J, Evaluation MSGMIRC Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 4.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L, Cardiac Resynchronization-Heart Failure Study I The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 5.Birnie DH, Ha A, Higginson L, Sidhu K, Green M, Philippon F, Thibault B, Wells G, Tang A. Impact of QRS morphology and duration on outcomes after cardiac resynchronization therapy: Results from the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT) Circ Heart Fail. 2013;6:1190–8. doi: 10.1161/CIRCHEARTFAILURE.113.000380. [DOI] [PubMed] [Google Scholar]
- 6.Roque C, Trevisi N, Silberbauer J, Oloriz T, Mizuno H, Baratto F, Bisceglia C, Sora N, Marzi A, Radinovic A, Guarracini F, Vergara P, Sala S, Paglino G, Gulletta S, Mazzone P, Cireddu M, Maccabelli G, Della Bella P. Electrical storm induced by cardiac resynchronization therapy is determined by pacing on epicardial scar and can be successfully managed by catheter ablation. Circ Arrhythm Electrophysiol. 2014;7:1064–9. doi: 10.1161/CIRCEP.114.001796. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JG, Abraham WT, Linde C, Gold MR, Young JB, Claude Daubert J, Sherfesee L, Wells GA, Tang AS. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. 2013;34:3547–56. doi: 10.1093/eurheartj/eht290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–24. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 9.Rumsfeld JS. Health status and clinical practice: when will they meet? Circulation. 2002;106:5–7. doi: 10.1161/01.cir.0000020805.31531.48. [DOI] [PubMed] [Google Scholar]
- 10.Medicine IIo. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 11.Tsevat J, Weeks JC, Guadagnoli E, Tosteson AN, Mangione CM, Pliskin JS, Weinstein MC, Cleary PD. Using health-related quality-of-life information: clinical encounters, clinical trials, and health policy. J Gen Intern Med. 1994;9:576–82. doi: 10.1007/BF02599287. [DOI] [PubMed] [Google Scholar]
- 12.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL, Resynchronization-Defibrillation for Ambulatory Heart Failure Trial I Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–95. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 13.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC, Schroeder JS, Liem LB, Hall S, Wheelan K, Multicenter InSync ICDRCETI Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–94. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 14.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C, Group RS Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–43. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. Journal of the American College of Cardiology. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 16.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J. 1992;124:1017–25. doi: 10.1016/0002-8703(92)90986-6. [DOI] [PubMed] [Google Scholar]
- 17.Spertus JA, Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8:469–76. doi: 10.1161/CIRCOUTCOMES.115.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS, Cardiovascular Outcomes Research C Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–15. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Bennett SJ, Oldridge NB, Eckert GJ, Embree JL, Browning S, Hou N, Chui M, Deer M, Murray MD. Comparison of quality of life measures in heart failure. Nurs Res. 2003;52:207–16. doi: 10.1097/00006199-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Lund LH, Braunschweig F, Benson L, Stahlberg M, Dahlstrom U, Linde C. Association between demographic, organizational, clinical, and socio-economic characteristics and underutilization of cardiac resynchronization therapy: results from the Swedish Heart Failure Registry. Eur J Heart Fail. 2017 doi: 10.1002/ejhf.781. [DOI] [PubMed] [Google Scholar]
- 21.Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence-based heart failure therapies on mortality. Am Heart J. 2011;161:1024–30e3. doi: 10.1016/j.ahj.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J, 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–16. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 23.Bryant AR, Wilton SB, Lai MP, Exner DV. Association between QRS duration and outcome with cardiac resynchronization therapy: a systematic review and meta-analysis. J Electrocardiol. 2013;46:147–55. doi: 10.1016/j.jelectrocard.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM, Comparison of Medical Therapy P. Defibrillation in Heart Failure I Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 25.Woods B, Hawkins N, Mealing S, Sutton A, Abraham WT, Beshai JF, Klein H, Sculpher M, Plummer CJ, Cowie MR. Individual patient data network meta-analysis of mortality effects of implantable cardiac devices. Heart. 2015;101:1800–6. doi: 10.1136/heartjnl-2015-307634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zusterzeel R, Selzman KA, Sanders WE, Canos DA, O’Callaghan KM, Carpenter JL, Pina IL, Strauss DG. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patient-level data. JAMA Intern Med. 2014;174:1340–8. doi: 10.1001/jamainternmed.2014.2717. [DOI] [PubMed] [Google Scholar]
- 27.Dec GW. Leaning Toward a Better Understanding of CRT in Women*. Journal of the American College of Cardiology. 2014;64:895–897. doi: 10.1016/j.jacc.2014.06.1161. [DOI] [PubMed] [Google Scholar]
- 28.Zusterzeel R, Curtis JP, Canos DA, Sanders WE, Selzman KA, Pina IL, Spatz ES, Bao H, Ponirakis A, Varosy PD, Masoudi FA, Strauss DG. Sex-specific mortality risk by QRS morphology and duration in patients receiving CRT: results from the NCDR. J Am Coll Cardiol. 2014;64:887–94. doi: 10.1016/j.jacc.2014.06.1162. [DOI] [PubMed] [Google Scholar]
- 29.Olechowski B, Sands R, Zachariah D, Andrews NP, Balasubramaniam R, Sopher M, Paisey J, Kalra PR. Is cardiac resynchronisation therapy feasible, safe and beneficial in the very elderly? J Geriatr Cardiol. 2015;12:497–501. doi: 10.11909/j.issn.1671-5411.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer DB, Kennedy KF, Noseworthy PA, Buxton AE, Josephson ME, Normand SL, Spertus JA, Zimetbaum PJ, Reynolds MR, Mitchell SL. Characteristics and outcomes of patients receiving new and replacement implantable cardioverter-defibrillators: results from the NCDR. Circ Cardiovasc Qual Outcomes. 2013;6:488–97. doi: 10.1161/CIRCOUTCOMES.111.000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raphael C, Briscoe C, Davies J, Ian Whinnett Z, Manisty C, Sutton R, Mayet J, Francis DP. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007;93:476–82. doi: 10.1136/hrt.2006.089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett JA, Riegel B, Bittner V, Nichols J. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung. 2002;31:262–70. doi: 10.1067/mhl.2002.124554. [DOI] [PubMed] [Google Scholar]
- 33.Goldman L, Cook EF, Mitchell N, Flatley M, Sherman H, Cohn PF. Pitfalls in the serial assessment of cardiac functional status. How a reduction in “ordinary” activity may reduce the apparent degree of cardiac compromise and give a misleading impression of improvement. J Chronic Dis. 1982;35:763–71. doi: 10.1016/0021-9681(82)90087-x. [DOI] [PubMed] [Google Scholar]
- 34.Spertus JA, Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015 doi: 10.1161/CIRCOUTCOMES.115.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.St John Sutton M, Ghio S, Plappert T, Tavazzi L, Scelsi L, Daubert C, Abraham WT, Gold MR, Hassager C, Herre JM, Linde C, Group RErRiSlvdS Cardiac resynchronization induces major structural and functional reverse remodeling in patients with New York Heart Association class I/II heart failure. Circulation. 2009;120:1858–65. doi: 10.1161/CIRCULATIONAHA.108.818724. [DOI] [PubMed] [Google Scholar]
- 36.Gold MR, Daubert C, Abraham WT, Ghio S, St John Sutton M, Hudnall JH, Cerkvenik J, Linde C. The effect of reverse remodeling on long-term survival in mildly symptomatic patients with heart failure receiving cardiac resynchronization therapy: results of the REVERSE study. Heart Rhythm. 2015;12:524–30. doi: 10.1016/j.hrthm.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


