Abstract
Screening of a library of diverse heterocyclic scaffolds identified substituted quinolines as inhibitors of the human proteasome. The heterocyclic library was prepared via a novel titanium-catalyzed multicomponent coupling reaction, which rendered a diverse set of isoxazoles, pyrimidines, pyrroles, pyrazoles and quinolines. SAR of the parent lead compound indicated that hydrophobic residues on the benzo-moiety significantly improved potency. Lead compound 25 inhibits the chymotryptic-like proteolytic activity of the proteasome (IC50 5.4 μM), representing a new class of nonpeptidic, noncovalent proteasome inhibitors.
Keywords: Proteasome, Inhibitors, Noncovalent, Quinolines
1. Introduction
Proteins undergo constant proteolytic degradation to regulate intracellular processes and maintain biological homeostasis.1,2 During this process, redundant and misfolded proteins are tagged with ubiquitin, which marks them for proteolytic degradation by the 26S proteasome. The 26S proteasome is comprised of a barrel shaped 20S core particle (CP) that is capped by two 19S recognition particles (RPs).3 The 20S CP is a threonine protease that contains three distinct catalytic subunits (β5, β2 and β1) that exhibit chymotrypsin-like (CT-L), trypsin-like (T-L) and caspase-like (Casp-L) activity.4 The 19S regulatory particle recognizes the ubiquitin tagged proteins and is responsible for the unfolding and translocation of these substrates into the 20S proteolytic core chamber.5
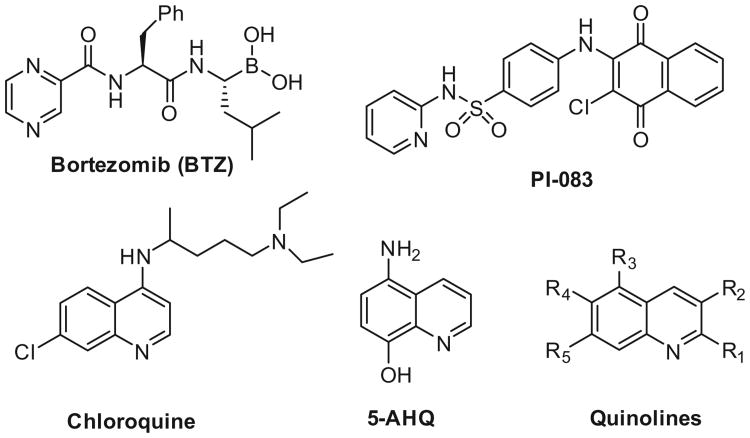
Modulation of proteasome function has emerged as an important approach to treat various diseases,6,7 and several proteasome inhibitors are clinically approved.8–10 Common themes among proteasome inhibitors are that they are protein mimics comprised of a peptide backbone containing an electrophilic warhead.11–13 The warhead forms a covalent bond with the 1N terminal Thr in the catalytic site(s) of the 20S core particle and abrogates its enzymatic activity. Thus, all these peptide based suicide inhibitors are competitive inhibitors that bind in the catalytic site(s) of the proteasome.11 The peptide-based suicide inhibitors bortezomib (Fig. 1, BTZ or Velcade) and carfilzomib (CFZ, Kyprolis) are FDA approved for the treatment of multiple myeloma (MM)14–17 and mantle cell lymphoma (MCL) and have validated the proteasome as an important clinical target.18–20
Figure 1.
Structures of covalent, peptidic proteasome inhibitors bortezomib, and noncovalent, nonpeptidic proteasome inhibitors PI-083, Chloroquine, 5AHQ and substituted quinolines.
These suicide inhibitors effectively block global protein proteolysis, which induces apoptosis, but also triggers a transcriptional feedback loop that results in the synthesis of new proteasome sub-units.21 In addition, peptidase cleavage induces the rapid systemic clearance of these inhibitors.10,22 Although the initial burst of inhibition is highly effective in the induction of apoptosis, the unfavorable pharmacodynamic properties of these peptide-based suicide inhibitors have restricted their use to blood cancers.
Noncovalent and nonpeptidic proteasome inhibition may limit some of these intrinsic pharmacokinetic drawbacks and may translate into a broader clinical profile.23,24 Relative to covalent binders, reports of noncovalent and nonpeptidic proteasome inhibitors are still scarce, but are gaining recognition as viable alternatives to peptide-based suicide inhibitors.11
Examples of nonpeptidic noncovalent proteasome modulators include the phakellins,25 oxadiazoles,26 hydroxyureas,24 imidazolines27,28 sulfone or piperazine agents,29 and tamoxifen derivatives.30
Substituted quinolines in particular represent a very interesting new class of proteasome inhibitors. Lawrence and co-workers discovered and optimized a novel class of hydrophthoquinone derivates as nonpeptidic, noncovalent proteasome inhibitors.31,32 These agents, exemplified by PI-083 (Fig. 1), demonstrated selectivity for cancer cells over non-transformed cells, which potentially broadens the range of anticancer activity.33 Recently, crystallographic screening of compounds revealed a sulfonamide substituted quinoline as a noncovalent inhibitor of only the β1/β2 subunits and not the β5 chymotryptic activity, thus identifying a new binding motif.34
Several quinolines have also been found to allosterically modulate proteasome activity.35 NMR studies found that the structurally similar anti-malaria drug chloroquine was found to allosterically modulate proteasome activity by binding to the α/β interface of the Thermoplasma proteasome.36 Similarly, 5-amino-8-hydroxyquinoline (5-AHQ, Fig. 1) was reported to be a non-competitive inhibitor of the human proteasome.37 Importantly, 5-AHQ was found effective against bortezomib resistant cell lines, thus exemplifying another advantage of using mechanistically distinct classes of proteasome inhibitors.27,38,39 It is therefore tempting to speculate that at least some of these noncovalent, nonpeptidic quinolines may occupy a common allosteric binding site.35
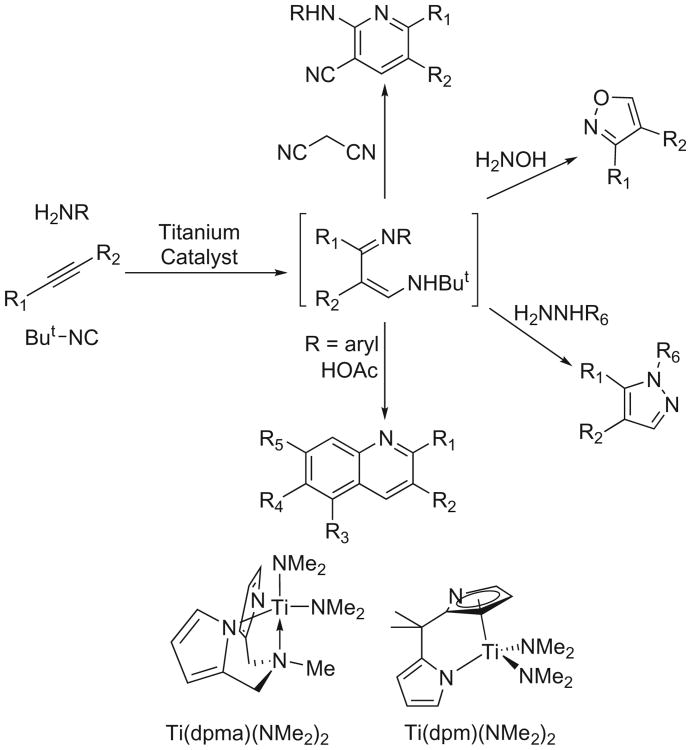
In our search for noncovalent nonpeptidic proteasome inhibitors, we screened several different classes of heterocyclic scaffolds for their ability to inhibit the chymotryptic activity of the human proteasome. The small diverse library was comprised of various aminocyanopyridines, isoxazoles, pyrazoles and quinolines that were prepared via a titanium-mediated multicomponent coupling reaction (Fig. 2). Of the compounds tested, only the quinolines exhibited low micromolar efficacies for 20S proteasome inhibition. Following the identification of the quinolines as initial hits in the in vitro screen, we characterized its mechanism as a mixed-type inhibition of proteasome modulation, which translated well in cell culture as indicated by the reduction of NF-κB mediated gene expression in a NF-κB-luc reporter assay in HeLa cells. In order to optimize the scaffolds activity, we modified five points of diversity (Fig. 1, R1–R5) along the scaffold backbone using the titanium catalyzed multicomponent coupling reaction.
Figure 2.
Titanium catalyzed multicomponent coupling reaction to form pyridines, isoxazoles, pyrazoles, and quinolines, as examples.
Herein, we report the synthesis of a novel class of quinoline-based scaffolds and their biological activity towards to 20S proteasome. Importantly, these scaffolds are structurally distinct from the hydro-phthoquinone and 5-AHQ, in that they do not contain, or can readily generate, an electrophilic benzoquinone moiety. In addition, the binding motif is likely different from the sulfonamide-based quinolines,34 as the compounds described herein are potent inhibitors of chymotryptic-like activity.
2. Results and discussion
2.1. Chemistry
A novel titanium-catalyzed 3-component coupling reaction was applied to generate a small library of diverse heterocyclic scaffolds.40–42 The titanium chemistry effectively adds an iminyl and amine group across the triple bond of an alkyne, iminoamination, to form unsymmetrical derivatives of 1,3-diimines. The 1,3-diimines, generated in situ, can then be applied to many different heterocyclic syntheses.40 The quinolines were prepared from the 3-component coupling products using a modified Combes synthesis catalyzed by acetic acid, which rendered highly substituted frameworks in a one-pot procedure (Fig. 2).43
The search for proteasome inhibition quickly narrowed to compounds containing the quinoline backbone. Due to the mechanism of the formation of the iminoamination products, the quinolines are all unsubstituted at the 4-position but can be substituted by a range of groups in other sites. The two catalysts employed for the syntheses are shown at the bottom of Figure 2. The ancillary ligands for titanium H2dpma and H2dpm are both prepared in a single step from pyrrole.44–46 The catalysts for this study were isolated as pure compounds before use; however, it is possible to generate the catalysts in situ from the protio-ligand and commercially available Ti(NMe2)4 as well.40 The applications of these two catalysts in iminoamination have been discussed elsewhere in detail.40 In short, more reactive Ti(dpm)(NMe2)2 is often used for more difficult internal alkyne substrates, while milder Ti(dpma)(NMe2)2 is often used with sensitive terminal alkynes to avoid potential side reactions. In addition, the two catalysts can direct regioselectivity for the substrates, which broadens the structural diversity.
The synthesis of some of the quinolines follows a similar one-pot synthesis as previously reported.43 Synthetic details for all new compounds can be found in the Supporting information.
2.2. Biological evaluation
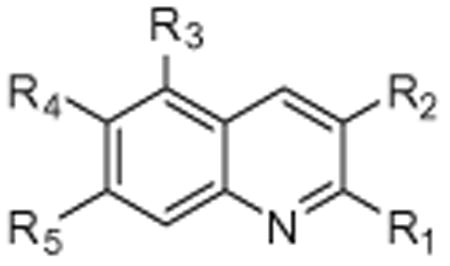
The initial diverse library of compounds was screened in vitro using purified human 20S proteasome and the fluorogenic peptide substrate, Suc-LLVY-AMC, as the substrate for CT-L activity.47 The rates of hydrolysis were monitored by fluorescence increase at 37 °C over 30 min, and the linear portion of the curves were used to calculate the IC50 values. Of the compounds tested, only some of the quinolines exhibited low micromolar efficacies for 20S proteasome inhibition (Fig. S1). Of the quinolines tested, quinoline 7 exhibited modest inhibition of the 20S chymotryptic activity with an IC50 of 14.4 μM and was therefore selected for further evaluation and optimization. Interestingly, it appeared that substitutions in the R1, R2, R3 and R5 positions were required to see any inhibition of proteasome activity (Table 1).
Table 1.
Structure and IC50 values of substituted quinones for inhibition of chymotryptic activity of the 20S proteasome. All IC50 values are averages of two independent experiments (each performed in triplicate)
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| R1 | R2 | R3 | R4 | R5 | IC50 (μM) | |
| BTZ (Fig. 1) | — | — | — | — | — | 0.0062 (±0.0003) |
| 1 | CH3 | H | H | H | H | >25 |
| 2 | H | CH3 | H | H | H | >25 |
| 3 | H |
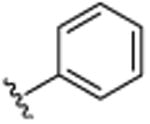
|
H | H | H | >25 |
| 4 | H |
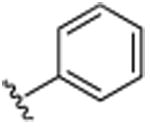
|
CH3 | H | CH3 | 23.6 (±1.9) |
| 5 | CH3 |
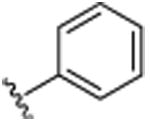
|
H | CH3 | H | 19.2 (±0.3) |
| 6 | CH3 |
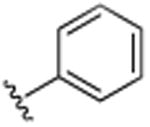
|
CH3 | H | CH3 | 15.3 (±2.4) |
| 7 | CH3 |
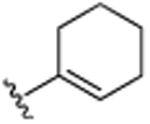
|
CH3 | H | CH3 | 14.4 (±0.5) |
Quinoline 7 was subsequently evaluated for its inhibition of the proteasome's tryptic (β2)-like and caspase (β1)-like activity in vitro using purified human 20S proteasome and the following fluorogenic peptide as substrates: Boc-LRR-AMC (substrate for T-L activity) and Z- LLE-AMC (substrate for casp-L activity).47 The data shows that the quinoline 7 inhibits the casp-L at a similar IC50 (IC50 17.7 μM) as the chymotryptic activity, but not the tryptic-L activities of the 20S catalytic core (IC50 > 25 μM, Fig. 3).
Figure 3.
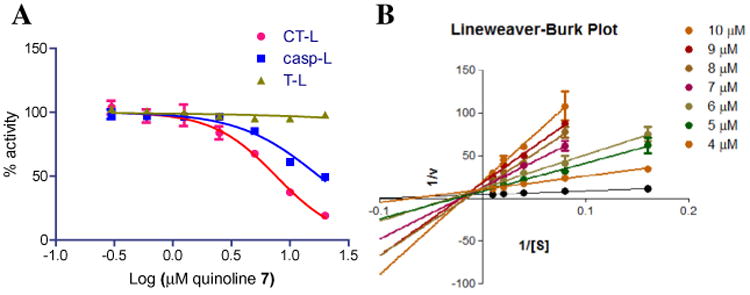
(A) Quinoline 7 inhibits the CT-L and Casp-L activity of the human proteasome. Fluorogenic substrates Suc-LLVY-AMC, Z-ARR-AMC and Z-LLE-AMC were used to measure CT-L, T-L and Casp-L activities of purified human 20S proteasome particles. The maximum increase in fluorescence per minute was used to calculate specific activities of each sample. (B) Lineweaver–Burk plot using SucLLVY-AMC consistent with a model of ‘mixed-type inhibition’. Undetectable proteasome activity was observed at the lowest amount of substrate thus these values are omitted in the L–B plot.
The mechanism by which quinoline 7 inhibits the proteasome was investigated using Michaelis-Menton analysis to determine of KM and Vmax and then further illustrated using a Lineweaver– Burk double reciprocal plot of the kinetic data. Kinetic analysis of CT-L activity of purified 20S particles indicate that when the substrate (Suc-LLVY-AMC) concentration was increased incrementally and measurements were taken at five different concentrations of compound 7 or vehicle, the Vmax of the CT-L activity decreases and the KM increases, with the increasing concentration of substrate (Fig. 3B). This is a pattern that is consistent with mixed-type inhibition,48 and is consistent with an allosteric-type modulation of proteasome activity, where binding of the quinoline 7 occurs at a site different from the active site, resulting in inhibition of enzyme activity.
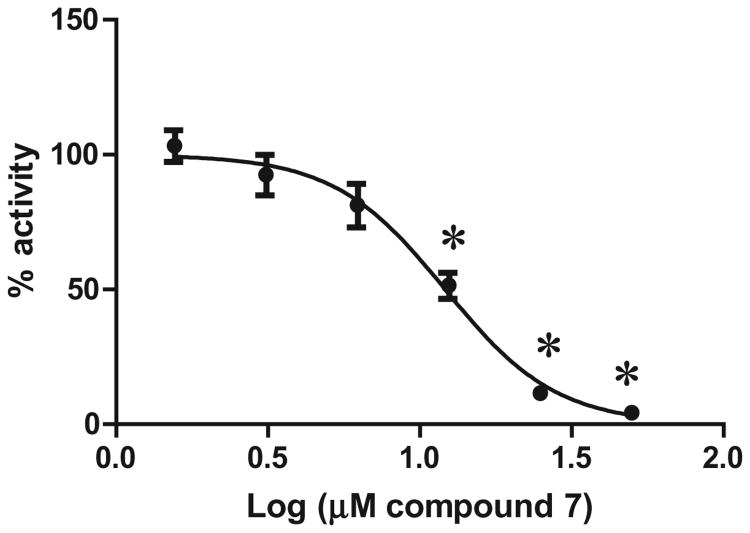
In order to evaluate whether the inhibition of proteasome activity translated in cell culture, we evaluated compound 7 for inhibition of NF-κB regulation. Inhibition of the proteasome affects multiple critical signaling pathways and the anti-cancer activity of proteasome inhibitors has been linked, in part, to their ability to inhibit the pro-inflammatory, anti-apoptotic NF-κB signaling path-way.49–51 The nuclear transcription factor, NF-κB is sequestered in the cytoplasm by the inhibitory protein κB, termed IκB. Activation of the NF-κB pathway by cytokines, such as TNF-α, results in the rapid ubiquitinylation and proteasomal degradation of IκB, which releases NF-κB for nuclear translocation and gene transcription.52 Proteasome inhibitors prevent IκB from proteolytic degrading and result in an accumulation of cytosolic ubiquitinylated IκB following TNF-α activation.53 In order to determine if quinoline 7 affects NF-κB mediated gene transcription, we evaluated quinoline 7 in HeLa NF-κB-luc cells.
Cell cultures were pretreated with vehicle (1% DMSO), the proteasome inhibitor MG-132 (positive control) or compound 7 (final concentrations were 50, 25, 12.5, 6.25, 3.13, 1.56 μM) for 30 min at 37 °C in 5% CO2. TNF-α was added to a final concentration of 25 ng/mL, and the samples were further incubated for 8 h at 37 °C in 5% CO2 and subsequently assayed for firefly luciferase production using the Steady-Glo luciferase reporter assay. The in vitro inhibition of the proteasome by quinoline 7 translated well in cell culture and prevented NF-κB mediated gene transcription with an IC50 value of 12.1 μM (Fig. 4) in a dose-response manner.
Figure 4.
NF-κB-luc HeLa cells were unstimulated/stimulated with 25 ng/mL TNF-α in the absence or presence of quinoline 7 in 1% DMSO. Fold-induction (%) of the Luciferase activity, normalized for all samples to TNF- α stimulation, is shown. Data shown is an average of 2 independent experiments. *Statistically significant from the vehicle + TNF control (one-way ANOVA n = 4).
Following the cellular validation of the observed in vitro proteasome activity, we moved to improve the potency and gain insight into the structural requirements for activity. For this, we expanded the scope of the 3-component coupling reaction to include several addition functional groups. Using the same assay conditions, several additional quinolines were evaluated for their ability to inhibit the chymotryptic activity of the proteasome.
First, we examined some changes in the R1 position (Table 2, 7–9), which rendered minor improvements in potency. Replacement of the cyclohexene with an isobutylene group (10), rendered an inactive compound, indicating the significance of the cyclohexene moiety. A significant gain (2-fold) in potency was seen using the cyclohexyl moiety (11), which provided our first single digit micro-Molar proteasome inhibitor. Activity decreased using a phenyl group in this position (Table 1, compound 6). A significant drop in activity was seen by replacing the R1 group of compound 6 with a hydrogen (Table 1, 4) or aryl moiety (12) indicating the need for small hydrophobic moieties around the quinoline scaffold.
Table 2. Structure and IC50 values of substituted quinolines for inhibition of chymotryptic activity of the 20S proteasome. All IC50 values are averages of two independent experiments (each performed in triplicate).
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| R1 | R2 | R3 | R4 | R5 | IC50 | |
| BTZ | — | — | — | — | — | 0.0062 (±0.0003) |
| 7 | CH3 |
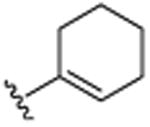
|
CH3 | H | CH3 | 14.4 (±0.5) |
| 8 | CH2CH3 |
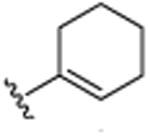
|
CH3 | H | CH3 | 19.9 (±0.7) |
| 9 | H |
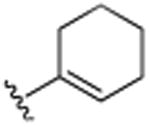
|
CH3 | H | CH3 | 13.8 (±1.3) |
| 10 | CH2CH3 |
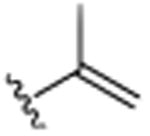
|
CH3 | H | CH3 | >25.0 |
| 11 | CH3 |
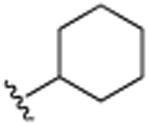
|
CH3 | H | CH3 | 8.2 (±1.2) |
| 12 |
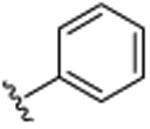
|
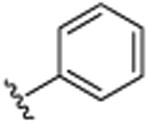
|
CH3 | H | CH3 | >25.0 |
| 13 | CH3 |
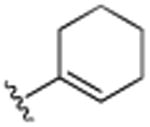
|
H | H | H | >25.0 |
| 14 | H |
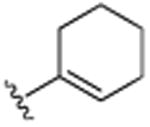
|
Cl | H | Cl | >25.0 |
| 15 | H |
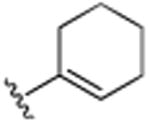
|
Br | H | Br | >25.0 |
| 16 | CH3 |
|
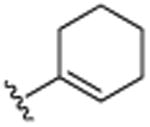
H | Br | H | 9.9 (±0.6) |
| 17 | CH3 |
|
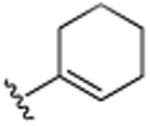
H | Cl | H | >25.0 |
| 18 | CH3 |
|
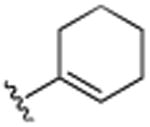
H | F | H | >25.0 |
| 19 | CH3 |
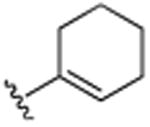
|
H | CH3 | H | 8.5 (±0.1) |
| 20 | CH3 |
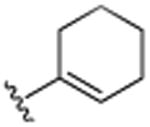
|
H | (CH2)3CH3 | H | 7.6 (±1.3) |
| 21 | H |
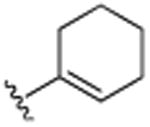
|
H | N(CH3)2 | H | 6.3 (±0.3) |
| 22 | CH3 |
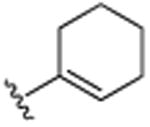
|
H | N(CH3)2 | H | 6.1 (±0.2) |
| 23 | CH2CH3 |
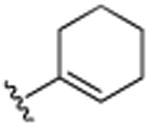
|
H | N(CH3)2 | H | 5.6 (±0.4) |
| 24 | CH3 |
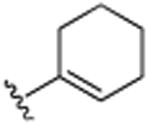
|
H |
|
H | 9.1 (±0.5) |
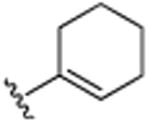
| 25 | CH3 |
|
H |
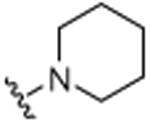
|
H | 5.4 (±0.1) |
| 26 | CH3 |
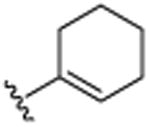
|
H | OCH3 | H | >25.0 |
| 27 | CH3 |
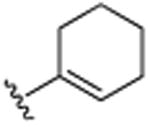
|
OCH3 | OCH3 | OCH3 | 15.6 (±0.7) |
| 28 | H |
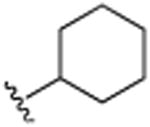
|
H | N(CH3)2 | H | 5.5 (±0.8) |
| 29 | CH3 |
|
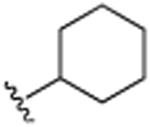
H | N(CH3)2 | H | 6.7 (±0.2) |
| 30 | CH3 |
|
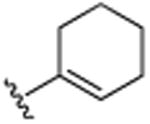
H | CH(CH3)2 | H | 7.8 (±0.1) |
| 31 | H | C(CH3)3 | H | N(CH3)2 | H | >25.0 |
| 32 | (CH2)3CH3 | H | H | N(CH3)2 | H | >25.0 |
| 33 | CH2CH3 | CH2CH3 | H | N(CH3)2 | H | 18.7 (±1.0) |
| 34 | CH3 |
|
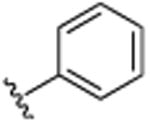
H | N(CH3)2 | H | 10.0 (±0.6) |
| 35 |
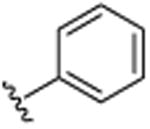
|
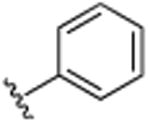
|
H | N(CH3)2 | H | >25 |
| 36 | CH3 |
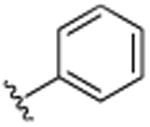
|
H | (CH2)3CH3 | H | 21.6 (±1.1) |
| 37 | CH3 |
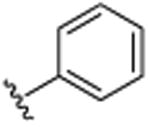
|
H | CH(CH3)2 | H | >25.0 |
| 38 | CH3 |
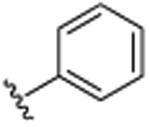
|
H | Br | H | >25.0 |
Next we evaluated the R3–R5 positions around the quinoline motif and compared these to our original lead (7), which contained a methyl in the R1 and the cyclohexene ring in the R2 position. Eliminating the R3 and R5 methyl groups, rendered inactive compounds (13), indicating the need of hydrophobic groups in these domains. Not even the lipophilic chloride (14) or bromides (15) in the R3 and R5 positions were able to restore the activity. However, by increasing the lipophilic bulk in the R4 position, Br (16) > Cl (17) = F (18) the activity was restored, indicative of a critical hydrophobic binding interaction of the benzo-portion of the quinoline scaffold. This was further supported by the positioning of a methyl in the R4 positions (compound 19) which restored much of the activity. These findings indicated to us that the binding pocket readily accepts larger hydrophobic moieties in the R3–R5 area of the quinoline scaffold.
This was further confirmed by the incorporation of a hydrophobic butyl group in the R4 position, which exhibited excellent activity (20, IC50 7.6 μM). Similarly, the dimethylamino moiety in the R4 position (22) successfully encumbers the hydrophobic pockets occupied by the two methyl groups in the R3 and R5 positions in compound 7. As seen previously, minor changes in potency were seen following changes in the R1 position (compounds 21–23). The incorporation of piperidinyl group (25) further improved activity with an IC50 of 5.4 μM The replacement of the dimethylamino group by the smaller methoxy moiety (26) eliminated all activity, which was partially restored by additional neighboring methoxy-groups (27). Considering the lack of activity of 26, this restoration of activity is likely due to positioning of the two methyl groups of dimethylamine of compound 22 in two hydrophobic binding pockets, rather than a possible hydrogen accepting role. This was further confirmed with compound 30, which contains an isopropyl moiety instead of the dimethylamino moiety and still retains much of its activity (IC50 of 7.8 μM). Considering the apparent requirement of the alkyl groups in the R3 and R5 positions in 7, it is likely that the two methyl groups on dimethylamine 22 occupy similar pockets given the close special proximity to the R3 and R5 position. As anticipated based on the activity of fully saturated cyclohexyl (11), compounds 28–30 all exhibit potent activities with IC50's in the 5–7 μM range. Further functionalization around the R1 and R2 position (31–38), did not render more active analogues, although the trends of proteasome activity followed our earlier findings.
The lead compound, quinoline 25, was subsequently evaluated for its inhibition of the proteasome's tryptic (β2)-like and caspase (β1)-like activity in vitro using purified human 20S proteasome. Similar to quinoline 7 (Fig. 3A), compound 25 also inhibited caspase-like activity (IC50 10.9 μM), but not the tryptic-like activity (Suppl. Fig. S6).
3. Conclusion
In conclusion, new classes of small molecule proteasome inhibitors are direly needed considering the inevitable resistance to current drugs and therapeutic limitations in the clinic. We described herein a novel class of noncovalent nonpeptidic proteasome inhibitors. The in vitro activity of our parent scaffold 7 translated well in cell culture, where quinoline 7 prevents NF-κB mediated gene expression in HeLa cells following TNF-α stimulation, consistent with a mechanism of proteasome inhibition. Our SAR study identified several domains that were found to be critical for the activity of this new lead template. Quinoline 25 was found to be the most active analogue in this series and inhibited the chymotryptic activity of the 20S proteasome with an IC50 of 5.4 μM This potency is similar to the noncovalent proteasome inhibitors, PI-083 (IC50 ∼ 1.0 μM),32 5-AHQ (IC50 ∼ 0.6–5 μM),37 and a significant improvement over the millimolar activity of chloroquine.36 Thus, these studies describe a novel class of quinoline-based proteasome inhibitors. Further optimization of this new template is currently under investigation in our laboratories as well as the evaluation of the potential clinical significance of this new nonpeptidic, noncovalent class of proteasome inhibitors.
4. Experimental section
All manipulations of air sensitive compounds were carried out in an Mbraun drybox under a purified nitrogen atmosphere. Toluene was purified by sparging with dry N2 and water was removed by running through activated alumina systems purchased from Solv-Tex. 1H, 13C and 19F spectra were recorded on VXR-500 spectrometers. Melting points are uncorrected and measured on a Mel-Temp II apparatus (Laboratory Devices Inc, USA) with a mercury thermometer in an open capillary tube. Ti(NMe2)2dpma and Ti (NMe2)2dpm were made following the literature procedures.44–46 Ti(NMe2)2dpm was used for all of the quinolines synthesized, with the exceptions 31 and 32 in which Ti(NMe2)2dpma was used. tert-Butylisonitrile was made according to the literature procedure54 and purified by distillation under dry nitrogen but it may also be purchased from Sigma Aldrich. Hexanes and ethyl acetate were purchased from Mallinckrodt chemicals and used as received. Alkynes were purchased either from Sigma Aldrich or from GFC chemicals and were dried/distilled from CaO under dry nitrogen before use. Amines were purchased from Sigma Aldrich, dried over KOH and distilled under nitrogen. Palladium(II) acetate, potassium tert-butoxide and 2-(dicyclohexylphosphino)biphenyl (97%) were also purchased from Sigma Aldrich and used as received. 2-methylquinoline (1), 3-methylquinoline (2), were purchased from TCI America. 3-phenylquinoline (3) was synthesized through via literature procedure.55 3-cyclohexenyl-2,5,7-trimethylquino-line (7), 2,5,7-trimethyl-3-phenylquinoline (6), 5,7-dimethyl-2, 3-diphenylquinoline (12), 2-methyl-3-phenyl-6-(N,N-dimethy-lamino)quinoline (34) were synthesized via the literature procedure.43
4.1. General procedure for the synthesis of quinolines (1–39)
In a nitrogen filled glove box, a 10 mL pressure tube, equipped with a magnetic stir bar was loaded with the titanium catalyst (0.10 mmol) and dissolved in dry toluene (2 mL). The solution was loaded with the aniline derivative (1.0 mmol), alkyne derivative (1.0 mmol) and tert-butylisonitrile (1.5 mmol). The pressure tube was sealed with a Teflon screw cap, taken out of the dry box and heated at 100 °C for 24–48 h in a silicone oil bath. Volatiles were removed in vacuo, and glacial acetic acid (2 mL) was added. The mixture was then heated at 150 °C for 24 h. The pressure tube was then allowed to cool to room temperature, diluted in dichloromethane and neutralized with saturated NaHCO3 solution. The organic layer was further extracted with additional dichloro-methane, washed with brine, dried over NaSO4, filtered and concentrated in vacuo. The crude product was dry loaded onto alumina and purification was accomplished by column chromatography on neutral alumina using hexanes/ethyl acetate (9:1, v/v) as the eluent to provide the desired quinoline.
4.1.1. 5,7-Dimethyl-3-phenylquinoline (4)
Pale yellow solid 128 mg (55%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 2.51 (3H, s, CH3), 2.68 (3H, s, CH3), 7.23 (1H, s, Ar-H), 7.39–7.42 (1H, m, Ar-H, 7.48–7.53 (2H, m, Ar-H), 7.68–7.70 (2H, m, Ar-H), 7.74 (1H, s, Ar-H), 8.36–8.37 (1H, d, J = 2 Hz, Ar-H), 9.10 (1H, d, J = 2 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 18.5, 21.8, 125.3, 126.4, 127.4, 127.8, 129.1 129.6, 129.8, 132.6, 134.2, 138.4, 139.3, 147.9, 149.3. MS (EI): m/z 233 (M+). Anal. found (calcd): C, 87.42 (87.52); H, 6.52 (6.48); N, 6.06 (6.00). Mp: 76–78 °C.
4.1.2. 2,6-Dimethyl-3-phenylquinoline (5)
Pale yellow solid 158 mg (68%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 2.50 (3H, s, CH3), 2.63 (3H, s, CH3), 7.37–7.39 (3H, m, Ar-H), 7.40–7.43 (2H, m, Ar-H), 7.49–7.51 (2H, m, Ar-H), 7.83 (1H, s, Ar-H), 7.94–7.96 (1H, d, J = 2 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 21.5, 24.4, 126.2, 126.7, 127.4, 128.0, 128.3, 129.1, 131.5, 135.4, 135.6, 135.7, 140.0, 145.6, 156.2 MS (EI): m/z 233 (M+). Anal. found (calcd): C, 87.62 (87.52); H, 6.42 (6.47); N, 5.96 (6.00). Mp: 80–81 °C.
4.1.3. 2-Ethyl-3-cyclohexenyl-5,7-dimethylquinoline (8)
Light yellow oil 102 mg (38%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.51–1.61 (7H, m) 1.99–2.03 (2H, m, CH2), 2.14–2.16 (2H, m, CH2), 2.21 (3H, s, CH3), 2.31 (3H, s, CH3), 3.04–3.09 (2H, q, J = 8 Hz, CH2), 5.60–5.62 (1H, m, CH), 6.86 (1H, s, Ar-H), 7.85 (1H, s, Ar-H), 8.05 (1H, s, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 13.6, 18.0, 21.3, 22.0, 23.0, 25.3, 29.1, 30.8, 124.3, 126.7, 126.8, 128.4, 130.5, 133.3, 136.5, 137.9, 138.0, 148.2, 160.9. MS (EI): m/z 265 (M+). HRMS, found: m/z 265.1840; calcd for C19H23N+ 265.1830.
4.1.4. 3-Cyclohexenyl-5,7-dimethylquinoline (9)
Pale yellow solid 99 mg (42%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.69–1.71 (2H, m, CH2), 1.82–1.84 (2H, m, CH2), 2.26–2.28 (2H, m, CH2), 2.47 (3H, s, CH3), 2.48–2.52 (2H, m, CH2), 2.63 (3H, s, CH3), 6.29–6.30 (1H, m, CH), 7.17 (1H, s, Ar-H), 7.66 (1H, s, Ar-H), 8.07–8.08 (1H, d, J = 2 Hz, Ar-H), 8.94–8.95 (1H, d, J = 2 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 18.6, 21.7, 21.9, 22.9, 26.0, 27.3, 125.2, 126.1, 126.8, 126.9, 128.3, 129.5, 129.6, 133.9, 134.1, 134.3, 148.1. MS (EI): m/z 237 (M+). Anal. found (calcd): C, 86.22 (86.03); H, 7.69 (8.07); N, 5.82 (5.90). Mp: 58–59 °C.
4.1.5. 2-Ethyl-5,7-dimethyl-3-(prop-1-en-2-yl)quinoline (10)
Pale yellow solid 158 mg (62%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.34–1.37 (3H, t, J = 7.5 Hz, CH3), 2.12 (3H, s, CH3), 2.46 (3H, s, CH3), 2.57 (3H, s, CH3), 2.96–3.00 (2H, q, J = 8 Hz, CH2), 4.98–4.99 (1H, d, J=1 Hz, CH), 5.27–5.28 (1H, d, J = 1 Hz, CH), 7.08 (1H, s, Ar-H), 7.67 (1H, s, Ar-H), 7.89 (1H, s, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 14.1, 18.3, 21.6, 24.9, 29.1, 116.1, 123.8, 125.7, 128.4, 130.7, 133.5, 135.7, 138.4, 144.5, 147.5, 160.6. MS (EI): m/z 255 (M+). Anal. found (calcd): C, 85.32 (85.2); H, 8.53 (8.50); N, 6.15 (6.22).
4.1.6. 3-Cyclohexyl-2,5,7-trimethylquinoline (11)
3-Cyclohexenyl-2,5,7-trimethylquinoline (7) (60 mg, 0.024 mmol) was dissolved in 6 mL of ethanol and hydrogenated at low pressure, using a hydrogen ballon, over 10% palladium on carbon (100 mg) at room temperature (25 °C) for an hour. Purification was accomplished via filtration through neutral alumina followed by column chromatography on neutral alumina using hexanes/ethyl acetate (9:1, v/v), which afforded the desired compound as a pale white liquid 54 mg (90%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.42–1.49 (4H, m, CH2), 1.79–1.82 (2H, m, CH2), 1.89–1.95 (4H, m, CH2), 2.46 (3H, s, CH3), 2.61 (3H, s, CH3), 2.73 (3H, s, CH3), 2.79-2.81 (1H, m, CH), 7.10 (1H, s, Ar-H), 7.62 (1H, s, Ar-H), 7.96 (1H, s, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 18.5, 22.8, 26.2, 27.1, 29.7, 33.9, 40.1, 124.7, 125.4, 128.1, 128.4, 128.5, 133.5, 138.2, 143.3, 157.3. MS (EI): m/z 253 (M+). Anal. found (calcd): C, 85.29 (85.32); H, 9.23 (9.15); N. 5.48 (5.53).
4.1.7. 3-Cyclohexenyl-2-methylquinoline (13)
Yellow liquid 60 mg (28%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.68–1.72 (2H, m, CH2), 1.76–1.80 (2H, m, CH2), 2.17–2.23 (2H, m, CH2), 2.23–2.26 (2H, m, CH2), 2.67 (3H, s, CH3), 5.67–5.68 (1H, m, CH), 7.40–7.43 (1H, m, Ar-H), 7.58–7.61 (1H, m, Ar-H), 7.69–7.71 (1H, d, J = 9 Hz, Ar-H), 7.75 (1H, s, Ar-H), 7.96–7.98 (1H, d, J = 9 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 21.9, 22.9, 23.7, 25.4, 30.1, 125.6, 126.9, 127.1, 127.4, 128.2, 128.7, 134.3, 137.3, 138.1, 146.6, 157.6. MS (EI): m/z 223 (M+). Anal. found (calcd): C, 86.12 (86.06); H, 7.60 (7.67); N, 6.28 (6.27).
4.1.8. 5,7-Dichloro-3-cyclohexenyl-2-methylquinoline (14)
Brown solid 131 mg (45%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.70–1.73 (2H, m, CH2), 1.77–1.80 (2H, m, CH2), 2.18–2.23 (2H, m, CH2), 2.23-2.26 (2H, m, CH2), 2.65 (3H, s, CH3), 5.69–5.71 (1H, m, CH), 7.46–7.47 (1H, d, J = 2 Hz, Ar-H), 7.87–7.89 (1H, m, J = 3 Hz, Ar-H), 8.06 (1H, s, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 21.9, 22.8, 23.6, 25.4, 29.9, 123.6, 126.4, 126.7, 128.3, 130.9, 131.6, 133.6, 136.7, 139.3, 147.2, 159.9.
MS (EI): m/z 292 (M+). Anal. found (calcd): C, 65.81 (65.77); H, 5.20 (5.17); N, 4.72 (4.79). Mp: 56–58 °C.
4.1.9. 5,7-Dibromo-3-cyclohexenyl-2-methylquinoline (15)
Yellow solid 122 mg (32%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ=1.71–1.73 (2H, m, CH2), 1.78–1.80 (2H, m, CH2), 2.20–2.23 (2H, m, CH2), 2.24–2.26 (2H, m, CH2), 2.66 (3H, s, CH3), 5.71–5.72 (1H, m, CH), 7.80–7.81 (1H, d, J = 2 Hz, Ar-H), 8.01 (1H, s, Ar-H), 8.11–8.12 (1H, d, J= 2 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 21.9, 22.8, 23.5, 25.4, 29.9, 121.8, 121.9, 125.2, 128.4, 130.7, 132.1, 133.5, 136.7, 139.8, 147.4, 159.9. MS (EI): m/z 381(M+). Anal. found (calcd): C, 50.51 (50.43); H, 3.93 (3.97); N, 3.64 (3.68). Mp: 89–90 °C.
4.1.10. 6-Bromo-3-cyclohexenyl-2-methylquinoline (16)
Light brown oil 103 mg (34%) 1H NMR (C6D6, 500 MHz, 20 °C): δ= 1.45–1.54 (4H, m, CH2), 1.93–1.96 (4H, m, CH2), 2.60 (3H, s, CH3), 5.41–5.42 (1H, m, CH), 7.13 (1H, s, Ar-H), 7.38–7.40 (1H, dd, J = 2 Hz, 9 Hz, Ar-H), 7.58–7.59 (1H, d, J = 2 Hz, Ar-H), 7.88–7.90 (1H, d, J = 9 Hz, Ar-H). 13C NMR (C6D6, 125 MHz, 20 °C): δ = 21.9, 22.8, 23.6, 25.3, 29.7, 119.2, 127.2, 128.2, 129.2, 130.7, 131.8, 132.7, 137.1, 138.6, 145.7, 157.8. MS (EI): m/z 301 (M+).HRMS, found: m/z 301.0480; calcd for C16H16BrN+ 301.0466.
4.1.11. 6-Chloro-2-methyl-3-cyclohexenylquinoline (17)
Light yellow oil 54 mg (21%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ =1.72–1.75 (2H, quin, J= 11 Hz, CH2), 1.79–1.82 (2H, quin, J = 12 Hz, CH2), 2.21 (2H, m, CH2), 2.25 (2H, m, CH2), 2.67 (3H, s, CH3), 6.32 (1H, m CH), 7.54–7.57 (1H, dd, J = 2 Hz, 9 Hz, Ar-H), 7.69 (1H, s, Ar-H), 7.70–7.71 (1H, d, J = 3 Hz, Ar-H), 7.91–7.93 (1H, d, J = 9 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 21.9, 22.9, 23.7, 25.4, 30.0, 125.8, 127.6, 127.9, 129.6, 129.8, 131.2, 133.5, 136.9, 139.1, 144.9, 158.2. MS (EI): m/z 257 (M+). HRMS, found: m/z 257.0963; calcd for C16H16ClN+ 257.0971.
4.1.12. 3-Cyclohexenyl-6-fluoro-2-methylquinoline (18)
Brown oil 38 mg (15%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ=1.69–1.71 (2H, m, CH2), 1.72–1.78 (2H, m, CH2), 2.17–2.19 (2H, m, CH2), 2.20–2.25 (2H, m, CH2), 2.65 (3H, s, CH3), 5.67–5.68 (1H, m, CH), 7.29–7.31 (1H, dd, J = 3 Hz, 9 Hz, Ar-H), 7.34–7.38 (1H, ddd, J = 3 Hz, 9 Hz, 9 Hz, Ar-H), 7.70 (1H, s, Ar-H), 7.94–7.97 (1H, dd, J= 6 Hz, 10 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 21.9, 22.9, 23.5, 25.4, 30.0, 110.0–110.1 (d, JCF = 21Hz), 118.6–118.8 (d, JCF = 26 Hz), 127.4–127.5 (d, JCF= 10 Hz), 127.7, 130.5–130.6 (d, JCF = 9Hz), 133.7–133.8 (d, JCF = 5Hz), 137.0, 138.9, 143.7, 157.0–157.1 (d d, JCF = 3 Hz), 159.1. 19F NMR (CDCl3, 470 MHz, 20 °C): δ = 115.0 (m). MS (EI): m/z 241 (M+). Anal. Found (calcd): C, 79.59 (79.64); H, 6.65 (6.68); N, 5.75 (5.80).
4.1.13. 3-Cyclohexenyl-2,6-dimethylquinoline (19)
Brown oil 123 mg (52%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ =1.68–1.72 (2H, m, CH2), 1.75–1.78 (2H, m, CH2), 2.17–2.20 (2H, m, CH2), 2.22–2.24 (2H, m, CH2), 2.46 (3H, s, CH3), 2.64 (3H, s, CH3), 5.65–5.67 (1H, m, CH), 7.42–7.45 (2H, m, Ar-H), 7.66 (1H, s, Ar-H), 7.85–7.86 (1H, d, J = 9 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 21.4, 22.0, 22.9, 23.6, 25.4, 30.1, 126.0, 126.9, 127.3, 127.9, 130.9, 133.7, 135.3, 137.4, 138.1, 145.3, 156.6. MS (EI): m/z 237 (M+). Anal. found (calcd): C, 86.04 (86.03); H, 8.09 (8.07); N, 5.87 (5.90).
4.1.14. 6-Butyl-3-cyclohexenyl-2-methylquinoline (20)
Viscous yellow oil 87 mg (32%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 0.94–0.96 (3H, t, J = 7 Hz, CH3), 1.35–1.43 (2H, sext, J = 15 Hz, CH2), 1.65–1.71 (2H, quin, J =15 Hz, CH2), 1.71–1.76 (2H, quin, J = 11 Hz, CH2), 1.79–1.84 (2H, quin, J =10 Hz, CH2), 2.22 (2H, m, CH2), 2.27 (2H, m, CH2), 2.68 (3H, s, CH3), 2.76–2.79 (2H, t, J = 8 Hz, CH2), 5.70 (1H, s, CH), 7.48–7.50 (2H, m, Ar-H), 7.73 (1H, s, Ar-H) 7.91–7.93 (1H, d, J = 9 Hz, Ar-H). 13C NMR(CDCl3, 125 MHz, 20 °C): δ =14.0, 22.1, 22.3, 23.0, 23.6, 25.5, 30.2, 33.5, 35.6, 125.5, 126.9, 127.3, 127.9, 130.4, 134.0, 137.4, 138.1, 140.3, 145.4, 156.7. MS (EI): m/z 279 (M+). HRMS, found: m/z 280.2062; calcd for C20H26N+ 280.2065.
4.1.15. 3-Cyclohexenyl-6-(N,N-dimethylamino)quinoline (21)
Viscous yellow oil 97 mg (38%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.41–1.46 (2H, m, CH2), 1.52–1.57 (2H, m, CH2), 1.95–1.97 (2H, m, CH2), 2.23–2.4 (2H, m, CH2), 2.51 (6H, s, N(CH3)2), 6.08–6.1 (1H, m, CH), 6.64–6.65 (1H, d, J = 3 Hz, Ar-H), 6.95–6.97 (1H, dd, J = 3Hz, 9 Hz, Ar-H), 7.67–7.69 (1H, d, J = 2Hz, Ar-H), 8.26–8.28 (1H, d, J = 9 Hz, Ar-H), 9.03–9.04 (1H, d, J = 2 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 22.0, 22.8, 25.8, 26.9, 39.9, 105.3, 118.5, 125.9, 128.2, 129.6, 130.1, 134.3, 135.0, 142.1, 144.9, 148.6. MS (EI): m/z 252 (M+). HRMS, found: m/z 252.1634; calcd for 252.1626.
4.1.16. 3-Cyclohexenyl-2-methyl-6-(N,N-dimethylamino)quinoline (22)
Brown solid 117 mg (44%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.72–1.74 (2H, m, CH2), 1.75–1.78 (2H, m, CH2), 2.22–2.24 (2H, m, CH2), 2.25–2.29 (2H, m, CH2), 2.64 (3H, s, CH3), 3.04 (6H, s, N(CH3)2), 5.68–5.70 (1H, m, CH), 6.78–6.79 (1H, d, J= 3 Hz, Ar-H), 7.29–7.32 (1H, dd, J = 3 Hz, 9 Hz, Ar-H), 7.63 (1H, s, Ar-H), 7.86–7.88 (1H, d, J = 9 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 22.1, 23.0, 23.2, 25.4, 30.1, 40.8, 105.2, 118.8, 126.8, 128.2, 128.8, 132.7, 137.7, 138.3, 140.8, 148.2, 153.1. MS (EI): m/z 266 (M+). Anal. found (calcd): C, 81.14 (81.16); H, 8.40 (8.32); N, 10.46 (10.52). Mp: 81–83 °C.
4.1.17. 3-Cyclohexenyl-2-ethyl-6(N,N-dimethylamino)quinoline (23)
Viscous light yellow oil 109 mg (39%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.52–1.60 (7H, m), 1.98–2.01 (2H, m, CH2), 2.12–2.17 (2H, m, CH2), 2.52 (6H, s, N(CH3)2), 3.03–3.07 (2H, quart, J = 7 Hz, CH2), 5.59 (1H, m), 6.65 (1H, d, J = 3 Hz, Ar-H), 6.98–7.00 (1H, dd, J =3 Hz, 9 Hz, Ar-H), 7.50 (1H, s, Ar-H), 8.20–8.22 (1H, d, J = 9 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 13.7, 22.1, 23.1, 25.4, 28.9, 30.7, 40.1, 105.1, 118.7, 126.3, 128.2, 128.4, 129.7, 132.7, 138.0, 141.9, 148.0, 157.2. MS (EI): m/z 280 (M+). HRMS, found: m/z 280.1928; calcd for 280.1939.
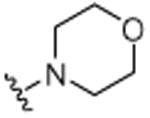
4.1.18. 3-Cyclohexenyl-2-methyl-6-morpholinylquinoline (24)
A pressure tube was loaded with Pd(OAc)2 (0.4 mg, 2 nmol), 2- (dicyclohexylphosphino)biphenyl (1.4 mg, 4 nmol), and KOtBu (53 mg, 48 mmol) under nitrogen atmosphere. Anhydrous toluene was added followed by 6-bromo-3-cyclohexenyl-2-methylquinoline (120 mg, 40 mmol) and morpholine (41 μL, 48 mmol). The tube was sealed, and then the mixture was stirred for 18 h at 110 °C. After cooling, the mixture was diluted with dichloromethane (20 mL) and washed with water (20 mL), and then brine (20 mL). The organic phase was dried over MgSO4, and then the solvent was removed in vacuo. Purification was accomplished by column chromatography on neutral alumina. The eluent was hexanes/ethyl acetate (19:1, v/v), which afforded the desired compound as a viscous brown oil 63 mg (51%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.68–1.78 (4H, m, CH2), 2.18–2.22 (4H, m, CH2), 2.62 (3H, s, CH3), 3.21–3.23 (4H, m, NCH2), 3.85–3.89 (4H, m, OCH2), 5.63–5.67 (1H, m, CH), 6.94–6.95 (1H, d, J = 3 Hz, Ar-H), 7.63 (1H, s, Ar-H), 7.88–7.90 (2H, d, J= 5 Hz, Ar-H), 8.00–8.02 (2H, d, J = 5 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 22.0, 23.1, 25.4, 29.6, 30.1, 49.6, 66.8, 121.5, 127.2, 127.8, 128.2, 128.8, 129.6, 132.9, 133.5, 138.5, 148.8, 154.7. MS (EI): m/z 308 (M+).Anal. found (calcd): C, 77.81 (77.89); H, 7.80 (7.84); N, 9.01 (9.08).
4.1.19. 3-Cyclohexenyl-2-methyl-6-piperidinylquinoline (25)
A pressure tube was loaded with Pd(OAc)2 (0.3 mg, 1.6 nmol), 2-(dicyclohexylphosphino)biphenyl (1.1 mg, 3.3 nmol), and KOtBu (44 mg, 39 mmol) under nitrogen atmosphere. Anhydrous toluene was added followed by 6-bromo-3-cyclohexenyl-2-methylquino-line (100 mg, 33 mmol) and piperidine (40 μL, 40 mmol). The tube was sealed, and then the mixture was stirred for 18 h at 110 °C. After cooling, the mixture was diluted with dichloromethane (20 mL) and washed with water (20 mL), and then brine (20 mL). The organic phase was dried over MgSO4, and then the solvent was removed in vacuo. Purification was accomplished by column chromatography on neutral alumina. The eluent was hexane/ethyl acetate (19:1, v/v), which afforded the desired compound as a viscous brown oil 42 mg (42%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.56–1.60 (2H, m, CH2), 1.61–1.78 (8H, m, CH2), 2.17–2.23 (4H, m, CH2), 2.61 (3H, s, CH3), 3.21–3.23 (4H, m, NCH2), 5.64–5.65 (1H, m, CH), 6.94–6.95 (1H, d, J = 3 Hz, Ar-H), 7.51–7.54 (1H, m, Ar-H), 7.60 (1H, s, Ar-H), 7.82–7.84 (2H, d, J= 10 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 22.0, 23.0, 23.2, 24.3, 25.4, 25.7, 30.1, 50.8, 128.3, 128.6, 129.6, 130.0, 132.9, 133.3, 137.6, 138.2, 149.7, 154.2, 166.5. MS (EI): m/z 306 (M+). Anal. found (calcd): C, 82.36 (82.31); H, 8.49 (8.55); N, 9.15 (9.14).
4.1.20. 3-Cyclohexenyl-6-methoxy-2-methylquinoline (26)
Brown solid 108 mg (43%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.72–1.75 (2H, m, CH2), 1.80–1.82 (2H, m, CH2), 2.22–2.23 (2H, m, CH2), 2.25–2.28 (2H, m, CH2), 2.66 (3H, s, CH3), 3.89 (3H, s, OCH3), 5.69–5.70 (1H, m, CH), 7.00–7.01 (1H, d, J = 3 Hz, Ar-H), 7.27–7.30 (1H, dd, J = 3 Hz, 9 Hz, Ar-H), 7.69 (1H, s, Ar-H), 7.89–7.91 (1H, d, J = 9 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 22.0, 22.9, 23.3, 25.4, 30.1, 55.4, 104.8, 121.1, 127.2, 127.7, 129.7, 133.4, 137.4, 138.4, 142.7, 154.9, 157.1. MS (EI): m/z 253(M+). Anal. found (calcd): C, 80.49 (80.60); H, 7.60 (7.56); N, 5.57 (5.53). Mp: 50–52 °C.
4.1.21. 3-Cyclohexenyl-5,6,7-trimethoxy-2-methylquinoline (27)
Viscous light yellow oil 131 mg (42%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.71–1.76 (2H, m, CH2), 1.79–1.84 (2H, m, CH2), 2.23 (2H, m, CH2), 2.27 (2H, m, CH2), 2.65 (3H, s, CH3), 3.97 (3H, s, OCH3), 3.99 (3H, s, OCH3), 4.06 (3H, s, OCH3), 5.69 (1H, s, Ar-H), 7.20 (1H, s, Ar-H), 7.98 (1H, s, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 22.0, 23.0, 23.4, 25.5, 30.3, 56.1, 61.2, 61.6, 103.2, 117.8, 127.4, 129.0, 136.0, 137.5, 140.3, 144.3, 146.8, 155.4, 156.8. MS (EI): m/z 313 (M+). HRMS, found: m/z 314.1763; calcdfor 314.1756. Mp: 93–95 °C.
4.1.22. 3-Cyclohexyl-6-(N,N-dimethylamino)quinoline (28)
3-cyclohexenyl-6-(N,N-dimethylamino)quinoline (21) (80 mg, 0.32 mmol) was dissolved in 6 mL of ethanol and hydrogenated at low pressure, using a hydrogen ballon, over 10% palladium on carbon (120 mg) at room temperature (25 °C) overnight. Purification was accomplished via filtration through neutral alumina followed by column chromatography on neutral alumina using hexanes/ethyl acetate (9:1, v/v), which afforded the desired compound as a viscous light yellow oil 75 mg (93%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.07–1.11 (1H, m, CH2), 1.18–1.22 (2H, m, CH2), 1.27–1.33 (2H, m, CH2), 1.58–1.60 (1H, m, CH2), 1.64–1.68 (2H, m, CH2), 1.74–1.77 (2H, m, CH2), 2.33–2.38 (1H, m, CH), 2.52 (6H, s, N (CH3)2), 6.65–6.66 (1H, d, J = 3 Hz, Ar-H), 6.97–6.99 (1H, dd, J = 3 Hz, 9 Hz, Ar-H), 7.52 (1H, d, J = 2 Hz, Ar-H), 8.26–8.28 (1H, d, J = 9 Hz, Ar-H), 8.71–8.72 (1H, d, J = 2 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 26.0, 26.7, 34.0, 40.0, 42.0, 105.1, 118.4, 129.8, 128.9, 130.2, 140.2, 142.1, 147.4, 148.4. MS (EI): m/z 254(M+). HRMS, found: m/z 254.1774; calcd for 254.1783.
4.1.23. 3-Cyclohexyl-2-methyl-6-(N,N-dimethylamino)quinoline (29)
2-Methyl-3-cyclohexenyl-6-(N,N-dimethylamino)quinoline (22) (100 mg, 0.38 mmol) was dissolved in 6 mL of ethanol and hydrogenated at low pressure, using a hydrogen ballon, over 10% palladium on carbon (150 mg) at room temperature (25 °C) over-night. Purification was accomplished via filtration through neutral alumina followed by column chromatography on neutral alumina using hexanes/ethyl acetate (9:1, v/v), which afforded the desired compound as a viscous yellow oil 98 mg (97%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.16–1.33 (4H, m, CH2), 1.63-1.79 (4H, m, CH2), 2.55 (6 H, s, CH3), 2.71 (3H, s, CH3), 6.72–6.73 (1H, d, J = 3 Hz, Ar-H), 6.99–7.01 (1H, dd, J = 3 Hz, 9 Hz, Ar-H), 7.63 (1H, s, Ar-H), 8.20–8.22 (1H, d, J = 9 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 25.6, 26.3, 27.0, 33.7, 39.9, 40.2, 105.3, 118.4, 129.0, 129.5, 129.6, 139.2, 141.3, 148.0, 153.4. MS (EI): m/z 268 (M+). HRMS, found: m/z 268.1934; calcd for 268.1939.
4.1.24. 3-Cyclohexenyl-6-isopropyl-2-methylquinoline (30)
Viscous yellow oil 112 mg (43%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.32–1.34 (6H, d, J = 7 Hz, CH3), 1.71–1.75 (2H, quin, J=11 Hz, CH2), 1.78–1.83 (2H, quin, J=12Hz, CH2), 2.21 (2H, m, CH2), 2.26 (2H, m, CH2), 2.68 (3H, s, CH3), 3.02–3.11 (1H, sept, J = 7 Hz, CH), 5.69 (1H, s, CH), 7.53 (1H, s, Ar-H), 7.53–7.55 (1H, app d, J = 9 Hz, Ar-H), 7.75 (1H, s, Ar-H), 7.94–7.96 (1H, d, J = 9 Hz, Ar-H). 13C NMR (C6D6, 125 MHz, 20 °C): δ = 22.0, 23.0, 23.5, 23.7, 25.3, 30.0, 34.0, 123.3, 126.8, 127.1, 128.3, 129.0, 133.6, 137.7, 137.8, 145.7, 146.5, 156.3. MS (EI): m/z 265 (M+).HRMS, found: m/z 266.1907; calcd for 266.1909.
4.1.25. 3-tert-Butyl-6-(N,N-dimethylamino)quinoline (31)
Viscous light brown oil 55 mg (24%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.20 (9H, s, C(CH3)3), 2.53 (6H, s, N(CH3)2), 6.67–6.68 (1H, d, J = 3Hz, Ar-H), 6.98–7.01 (1H, dd, J = 3Hz, 9 Hz, Ar-H), 7.72–7.73 (1H, d, J = 3 Hz, Ar-H), 8.26–8.27 (1H, d, J = 9 Hz, Ar-H), 8.96–8.98 (1H, d, J = 2 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 30.6, 33.3, 40.0, 105.3, 118.4, 128.4, 129.5, 130.0, 141.5, 143.0, 146.0, 148.5. MS (EI): m/z 228 (M+). HRMS, found:m/z 228.1617; calcd for 228.1626.
4.1.26. 2-Butyl-6-(N,N-dimethylamino)quinoline (32)
Viscous brown oil 69 mg (30%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 0.86–0.89 (3H, t, J=7Hz, CH3), 1.32–1.39 (2H, sext, J = 7 Hz, CH2), 1.82–1.88 (2H, quin, J = 7 Hz, CH2), 2.50 (6H, s, N (CH3)2), 2.92-2.95 (2H, t, J = 7 Hz, CH2), 6.64–6.65 (1H, d, J = 3 Hz, Ar-H), 6.92–6.93 (1H, d, J = 8 Hz, Ar-H), 6.98–7.01 (1H, dd, J = 3 Hz, 9 Hz, Ar-H), 7.61–7.63 (1H, d, J = 8 Hz, Ar-H), 8.19–8.21 (1H, d, J = 9 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 13.9, 22.6, 31.9, 39.5, 40.0, 105.4, 119.1, 121.4, 128.1, 130.1, 133.8, 142.9, 148.0, 158.4. MS (EI): m/z 228 (M+). HRMS, found: m/z 228.1616; calcd for 228.1626.
4.1.27. 2,3-Diethyl-N,N-dimethylquinoline (33)
Light brown solid 74 mg (33%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.32–1.35 (3H, t, J = 7 Hz, CH3), 1.35–1.38 (3H, t, J = 7 Hz, CH3), 2.78–2.83 (2H, q, J= 7 Hz, CH2), 2.95–2.99 (2H, q, J = 7 Hz, CH2), 3.06 (6H, s, N(CH3)2), 6.79 (1H, d, J = 2.8 Hz, Ar-H), 7.29–7.31 (1H, dd, J = 3 Hz, 9 Hz, Ar-H), 7.71 (1H, s, Ar-H), 7.89–7.91 (1H, d, J = 9 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 13.9, 14.5, 25.1, 28.5, 41.9, 105.0, 118.6, 128.7, 128.9, 132.5, 135.2, 148.2, 158.8. MS (EI): m/z 228 (M+). HRMS, found: m/z 229.1712; calcd for 229.1705. Mp: 64–66 °C.
4.1.28. 2,3-Diphenyl-6-(N,N-dimethylamino)quinoline (35)
Dark yellow solid 90 mg (28%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 2.52 (1H, s, N(CH3)2), 6.63–6.64 (1H, d, J = 3 Hz, Ar-H), 7.01–7.06 (5H, m, Ar-H), 7.07–7.10 (2H, m, Ar-H), 7.19–7.21 (2H, m, Ar-H), 7.72–7.74 (3H, m, Ar-H), 8.30–8.32 (1H, d, J = 9 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 39.9, 104.6, 119.4, 126.7, 127.2, 127.53, 128.0, 128.9, 129.8, 130.4, 130.5, 134.7, 135.6, 141.2, 141.4, 142.3, 148.5, 153.9. MS (EI): m/z 324 (M+). HRMS, found:m/z 324.1642; calcd for 324.1626. Mp: 159–161 °C.
4.1.29. 6-Butyl-2-methyl-3-phenylquinoline (36)
Viscous yellow oil 101 mg (37%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 0.95–0.98 (3H, t, J=7Hz, CH3), 1.37–1.45 (2H, sext, J =10 Hz, CH2), 1.68–1.74 (2 J, quin, J =10 Hz, CH2), 2.67 (3H, s, CH3), 2.79–2.82 (2H, t, J = 5 Hz, CH2), 7.41–7.44 (3H, m, Ar-H), 7.47–7.50 (2H, m, Ar-H), 7.56–7.57 (2H, m, Ar-H), 7.91 (1H, s, Ar-H), 7.99–8.01 (1H, d, J = 9 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 14.0, 22.4, 24.4, 33.5, 35.6, 125.7, 126.8, 127.5, 128.1, 128.4, 129.2, 131.0, 135.6, 135.7, 140.1, 140.8, 145.8, 156.3. MS (EI): m/z 275 (M+). HRMS, found: m/z 276.1750;calcd for C20H22N+ 276.1752.
4.1.30. 6-Isopropyl-2-methyl-3-phenylquinoline (37)
Viscous yellow oil 143 mg (55%). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 1.35–1.37 (2H, d, J = 7 Hz, CH3), 2.67 (3H, s, CH3), 3.06–3.15 (1H, sept, J = 8 Hz, CH), 7.40–7.44 (3H, m, Ar-H), 7.47–7.50 (2H, m, Ar-H), 7.60 (1H, s, Ar-H), 7.61–7.64 (1H, dd, J = 9 Hz, Ar-H), 7.93 (1H, s, Ar-H), 8.01–8.03 (1H, d, J = 9 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 23.9, 24.5, 29.7, 34.1, 123.5, 126.8, 127.5, 128.2, 128.4, 129.2, 129.3, 135.6, 135.9, 140.1, 145.9, 146.7, 156.4. MS (EI): m/z 261 (M+). HRMS, found:m/z 262.1596; calcd for C19H20N+ 262.1593.
4.1.31. 6-Bromo-2-methyl-3-phenylquinoline (38)
Light tan solid 337 mg (26%, on 5 mmol scale). 1H NMR (CDCl3, 500 MHz, 20 °C): δ = 2.66 (3H, s, CH3), 7.39–7.41 (2H, m, Ar-H), 7.43–7.46 (1H, m, Ar-H), 7.48–7.52 (2H, m, Ar-H), 7.76–7.78 (1H, dd, J = 2 Hz, 9 Hz, Ar-H), 7.87 (1H, s, Ar-H), 7.93–7.95 (1H, d, J = 9 Hz, Ar-H), 7.95–7.96 (1H, d, J = 2 Hz, Ar-H). 13C NMR (CDCl3, 125 MHz, 20 °C): δ = 24.7, 119.7, 127.8, 128.0, 128.5, 129.1, 129.4, 130.2, 132.7, 134.9, 136.6, 139.4, 145.6, 158.0. MS (EI): m/z 297(M+). HRMS, found: m/z 298.0230; calcd for C16H13BrN+298.0231. Mp: 88–90 °C.
4.2. Biological assays
4.2.1. 20S Proteasomal activity measurement
The fluorogenic substrates Suc-LLVY-AMC, Z-ARR-AMC, and Z-LLE-AMC were used to measure CT-L, T-L and casp-L proteasome activities, respectively. Assays were carried out in black, clear bottom 96 well plates in a 200 μL reaction volume containing 1 nM of purified human 20S proteasome in 50 mM Tris-HCL pH 7.5 and 0.03% SDS containing 50 μM fluorogenic substrate at 37 °C. The rate of cleavage of fluorogenic peptide substrates was determined by monitoring the fluorescence of released aminomethylcoumarin using a SpectraMax M5e multiwall plate reader at an excitation wavelength of 380 nm and emission wavelength of 460 nm. Fluorescence was measured every minute over a period of 30 minutes and the maximum increase in fluorescence per minute was used to calculate specific activities of each sample.
4.2.2. NF-κB-luc reporter assay
HeLa NF-κB-luc cells (5.0 × 105 cells/mL) were seeded into a 96-well white opaque plate using DMEM medium supplemented with 5% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM sodium pyruvate, 0.2 mM L-glutamine, and 100 μg/mL hygromycin B. After 24 h the cell culture medium was replaced with DMEM medium supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin. Cell cultures were pretreated with vehicle (1% DMSO), 50 μM peptide aldehyde MG-132 (positive control) or quinoline (final concentrations were 50, 25, 12.5, 6.25, 3.13 and 1.56 μM) for 30 min at 37 °C in 5% CO2. TNF-α was added to a final concentration of 25 ng/mL and the samples were further incubated for 8 h at 37 °C in 5% CO2. Cells were assayed for firefly luciferase production using the Steady-Glo luciferase reporter assay (Promega, Madison, WI) according to manufacturer's protocol. The luminescence of each well was measured using a Veritas microplate luminometer. All reported data are the average of two independent experiments unless otherwise indicated. The data was analyzed using GraphPad Prism 4.0. The data was normalized to TNF-α activation and the EC50 values were calculated using the equation for the sigmodial curve for variable slope.
Supplementary Material
Acknowledgments
Financial support for this work was provided in part by the National Science Foundation CHE-1265738 (A.L.O.) and National Institutes of Health CA-142644-01 (J.J.T.).
Footnotes
Supplementary data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmc.2016.04.005.
References and notes
- 1.Murata S, Yashiroda H, Tanaka K. Nat Rev Mol Cell Biol. 2009;10:104. doi: 10.1038/nrm2630. [DOI] [PubMed] [Google Scholar]
- 2.Groll M, Huber R. Biochim Biophys Acta. 2004;1695:33. doi: 10.1016/j.bbamcr.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Nissan G, Sharon M. Biomolecules. 2014;4:862. doi: 10.3390/biom4030862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groll M, Heinemeyer W, Jager S, Ullrich T, Bochtler M, Wolf DH, Huber R. Proc Natl Acad Sci USA. 1999;96:10976. doi: 10.1073/pnas.96.20.10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, Bartunik HD, Huber R. Nature. 1997;386:463. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 6.Stintzing S, Lenz HJ. Clin Cancer Res. 2014;20:3064. doi: 10.1158/1078-0432.CCR-13-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petroski MD. BMC Biochem. 2008;9:S7. doi: 10.1186/1471-2091-9-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP. Curr Cancer Drug Targets. 2012;11:239. doi: 10.2174/156800911794519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zavrski I, Jakob C, Kaiser M, Fleissner C, Heider U, Sezer O. Recent Results Cancer Res. 2007;176:165. doi: 10.1007/978-3-540-46091-6_14. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Yang J, Kirk C, Fang Y, Alsina M, Badros A, Papadopoulos K, Wong A, Woo T, Bomba D, Li J, Infante JR. Drug Metab Dispos. 2013;41:230. doi: 10.1124/dmd.112.047662. [DOI] [PubMed] [Google Scholar]
- 11.Beck P, Dubiella C, Groll M. Biol Chem. 2012;393:1101. doi: 10.1515/hsz-2012-0212. [DOI] [PubMed] [Google Scholar]
- 12.Kisselev AF. Chem Biol. 2008;15:419. doi: 10.1016/j.chembiol.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Borissenko L, Groll M. Chem Rev. 2007;107:687. doi: 10.1021/cr0502504. [DOI] [PubMed] [Google Scholar]
- 14.Sterz J, von Metzler I, Hahne JC, Lamottke B, Rademacher J, Heider U, Terpos E, Sezer O. Expert Opin Invest Drugs. 2008;17:879. doi: 10.1517/13543784.17.6.879. [DOI] [PubMed] [Google Scholar]
- 15.Tobinai K. Int J Clin Oncol. 2007;12:318. doi: 10.1007/s10147-007-0695-5. [DOI] [PubMed] [Google Scholar]
- 16.Mateos MV, San Miguel J. Best Pract Res Clin Haematol. 2007;20:701. doi: 10.1016/j.beha.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Kanagasabaphy P, Morgan GJ, Davies FE. Curr Opin Invest Drugs. 2007;8:447. [PubMed] [Google Scholar]
- 18.Barr P, Fisher R, Friedberg J. Cancer Invest. 2007;25:766. doi: 10.1080/07357900701579570. [DOI] [PubMed] [Google Scholar]
- 19.Vink J, Cloos J, Kaspers GJ. Br J Haematol. 2006;134:253. doi: 10.1111/j.1365-2141.2006.06170.x. [DOI] [PubMed] [Google Scholar]
- 20.Nau KC, Lewis WD. Am Fam Physician. 2008;78:853. [PubMed] [Google Scholar]
- 21.Radhakrishnan SK, Lee CS, Young P, Beskow A, Chan JY, Deshaies RJ. Mol Cell. 2010;38:17. doi: 10.1016/j.molcel.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz R, Davidson T. Oncology (Williston Park) 2004;18:14. [PubMed] [Google Scholar]
- 23.Groll M, Huber R, Moroder L. J Pept Sci. 2009;15:58. doi: 10.1002/psc.1107. [DOI] [PubMed] [Google Scholar]
- 24.Gallastegui N, Beck P, Arciniega M, Huber R, Hillebrand S, Groll M. Angew Chem Int, Ed Engl. 2012;51:247. doi: 10.1002/anie.201106010. [DOI] [PubMed] [Google Scholar]
- 25.Beck P, Lansdell TA, Hewlett NM, Tepe JJ, Groll M. Angew Chem Int Ed, Engl. 2015 doi: 10.1002/anie.201410168. [DOI] [PubMed] [Google Scholar]
- 26.Ozcan S, Kazi A, Marsilio F, Fang B, Guida WC, Koomen J, Lawrence HR, Sebti SM. J Med Chem. 2013;56:3783. doi: 10.1021/jm400221d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lansdell TA, Hurchla MA, Xiang J, Hovde S, Weilbaecher KN, Henry RW, Tepe JJ. ACS Chem Biol. 2013;8:578. doi: 10.1021/cb300568r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azevedo L, Lansdell T, Ludwig J, Woloch D, Cogan D, Patten G, Kuszpit M, Fisk J, Mosey RA, Tepe JJ. J Med Chem. 2013;56:5974. doi: 10.1021/jm400235r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basse N, Montes M, Marechal X, Qin L, Bouvier-Durand M, Genin E, Vidal J, Villoutreix BO, Reboud-Ravaux M. J Med Chem. 2010;53:509. doi: 10.1021/jm9011092. [DOI] [PubMed] [Google Scholar]
- 30.Hasegawa M, Yasuda Y, Tanaka M, Nakata K, Umeda E, Wang Y, Watanabe C, Uetake S, Kunoh T, Shionyu M, Sasaki R, Shiina I, Mizukami T. Eur J Med Chem. 2014;71:290. doi: 10.1016/j.ejmech.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Ge Y, Kazi A, Marsilio F, Luo Y, Jain S, Brooks W, Daniel KG, Guida WC, Sebti SM, Lawrence HR. J Med Chem. 2012;55:1978. doi: 10.1021/jm201118h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence HR, Kazi A, Luo Y, Kendig R, Ge Y, Jain S, Daniel K, Santiago D, Guida WC, Sebti SM. Bioorg Med Chem. 2010;18:5576. doi: 10.1016/j.bmc.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazi A, Lawrence H, Guida WC, McLaughlin ML, Springett GM, Berndt N, Yip RM, Sebti SM. Cell Cycle. 2009;8:1940. doi: 10.4161/cc.8.12.8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck P, Reboud-Ravaux M, Groll M. Angew Chem Int, Ed Engl. 2015;54:11275. doi: 10.1002/anie.201505054. [DOI] [PubMed] [Google Scholar]
- 35.Gaczynska M, Osmulski PA. Antioxid Redox Signal. 2014;21:2286. doi: 10.1089/ars.2013.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sprangers R, Li X, Mao X, Rubinstein JL, Schimmer AD, Kay LE. Biochemistry. 2008;47:6727. doi: 10.1021/bi8005913. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Wood TE, Sprangers R, Jansen G, Franke NE, Mao X, Wang X, Zhang Y, Verbrugge SE, Adomat H, Li ZH, Trudel S, Chen C, Religa TL, Jamal N, Messner H, Cloos J, Rose DR, Navon A, Guns E, Batey RA, Kay LE, Schimmer AD. J Natl Cancer Inst. 2010;102:1069. doi: 10.1093/jnci/djq198. [DOI] [PubMed] [Google Scholar]
- 38.de Wilt LH, Jansen G, Assaraf YG, van Meerloo J, Cloos J, Schimmer AD, Chan ET, Kirk CJ, Peters GJ, Kruyt FA. Biochem Pharmacol. 2012;83:207. doi: 10.1016/j.bcp.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Huber EM, Heinemeyer W, Groll M. Structure. 2015 doi: 10.1016/j.str.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Odom AL, McDaniel TJ. Acc Chem Res. 2015 doi: 10.1021/acs.accounts.5b00280. [DOI] [PubMed] [Google Scholar]
- 41.Dissanayake AA, Odom AL. Chem Commun (Camb) 2012;48:440. doi: 10.1039/c1cc15809k. [DOI] [PubMed] [Google Scholar]
- 42.Swartz DL, 2nd, Staples RJ, Odom AL. Dalton Trans. 2011;40:7762. doi: 10.1039/c1dt10127g. [DOI] [PubMed] [Google Scholar]
- 43.Majumder S, Gipson KR, Odom AL. Org Lett. 2009;11:4720. doi: 10.1021/ol901855b. [DOI] [PubMed] [Google Scholar]
- 44.Harris SA, Ciszewski JT, Odom AL. Inorg Chem. 2001;40:1987. doi: 10.1021/ic001238r. [DOI] [PubMed] [Google Scholar]
- 45.Shi Y, Hall C, Ciszewski JT, Cao C, Odom AL. Chem Commun (Camb) 2003:586. doi: 10.1039/b212423h. [DOI] [PubMed] [Google Scholar]
- 46.Novak A, Blake AJ, Wilson C, Love JB. Chem Commun (Camb) 2002:2796. doi: 10.1039/b208751k. [DOI] [PubMed] [Google Scholar]
- 47.Gaczynska M, Osmulski PA. Methods Mol Biol. 2005;301:3. doi: 10.1385/1-59259-895-1:003. [DOI] [PubMed] [Google Scholar]
- 48.Segel IH. Enzyme Kinetics. John Wiley & Sons Inc; New York: 1975. [Google Scholar]
- 49.Lee SW, Kim JH, Park YB, Lee SK. Ann Rheum Dis. 2009;68:1761. doi: 10.1136/ard.2008.097709. [DOI] [PubMed] [Google Scholar]
- 50.Nencioni A, Grunebach F, Patrone F, Ballestrero A, Brossart P. Leukemia. 2007;21:30. doi: 10.1038/sj.leu.2404444. [DOI] [PubMed] [Google Scholar]
- 51.Tsukamoto S, Yokosawa H. Expert Opin Ther Targets. 2009;13:605. doi: 10.1517/14728220902866851. [DOI] [PubMed] [Google Scholar]
- 52.Napetschnig J, Wu H. Annu Rev Biophys. 2013;42:443. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sors A, Jean-Louis F, Pellet C, Laroche L, Dubertret L, Courtois G, Bachelez H, Michel L. Blood. 2006;107:2354. doi: 10.1182/blood-2005-06-2536. [DOI] [PubMed] [Google Scholar]
- 54.Gokel GW, Widera RP, Weber WP. Org Synth. 1976;55:232. [Google Scholar]
- 55.Li AH, Beard DJ, Coate H, Honda A, Kadalbajoo M, Kleinberg A, Laufer R, Mulvihill KM, Nigro A, Rastogi P, Sherman D, Siu KW, Steinig AG, Wang T, Werner D, Crew AP, Mulvihill MJ. Synthesis. 2010 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.