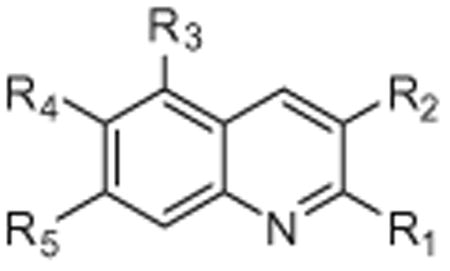
Table 2. Structure and IC50 values of substituted quinolines for inhibition of chymotryptic activity of the 20S proteasome. All IC50 values are averages of two independent experiments (each performed in triplicate).
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| R1 | R2 | R3 | R4 | R5 | IC50 | |
| BTZ | — | — | — | — | — | 0.0062 (±0.0003) |
| 7 | CH3 |
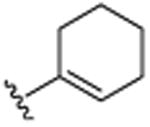
|
CH3 | H | CH3 | 14.4 (±0.5) |
| 8 | CH2CH3 |
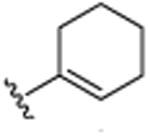
|
CH3 | H | CH3 | 19.9 (±0.7) |
| 9 | H |
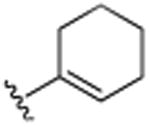
|
CH3 | H | CH3 | 13.8 (±1.3) |
| 10 | CH2CH3 |
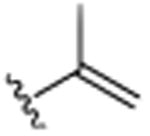
|
CH3 | H | CH3 | >25.0 |
| 11 | CH3 |
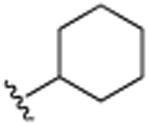
|
CH3 | H | CH3 | 8.2 (±1.2) |
| 12 |
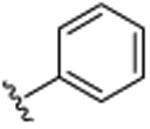
|
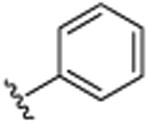
|
CH3 | H | CH3 | >25.0 |
| 13 | CH3 |
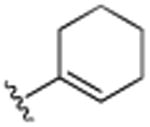
|
H | H | H | >25.0 |
| 14 | H |
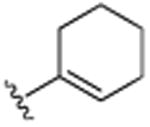
|
Cl | H | Cl | >25.0 |
| 15 | H |
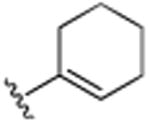
|
Br | H | Br | >25.0 |
| 16 | CH3 |
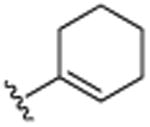
|
H | Br | H | 9.9 (±0.6) |
| 17 | CH3 |
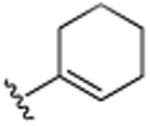
|
H | Cl | H | >25.0 |
| 18 | CH3 |
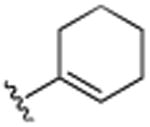
|
H | F | H | >25.0 |
| 19 | CH3 |
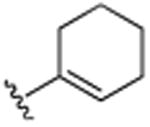
|
H | CH3 | H | 8.5 (±0.1) |
| 20 | CH3 |
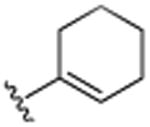
|
H | (CH2)3CH3 | H | 7.6 (±1.3) |
| 21 | H |
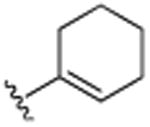
|
H | N(CH3)2 | H | 6.3 (±0.3) |
| 22 | CH3 |
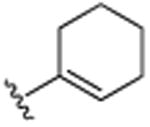
|
H | N(CH3)2 | H | 6.1 (±0.2) |
| 23 | CH2CH3 |
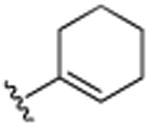
|
H | N(CH3)2 | H | 5.6 (±0.4) |
| 24 | CH3 |
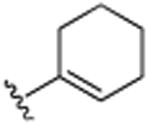
|
H |
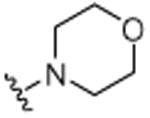
|
H | 9.1 (±0.5) |
| 25 | CH3 |
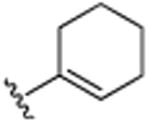
|
H |
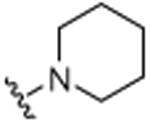
|
H | 5.4 (±0.1) |
| 26 | CH3 |
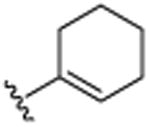
|
H | OCH3 | H | >25.0 |
| 27 | CH3 |
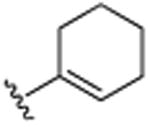
|
OCH3 | OCH3 | OCH3 | 15.6 (±0.7) |
| 28 | H |
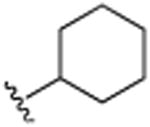
|
H | N(CH3)2 | H | 5.5 (±0.8) |
| 29 | CH3 |
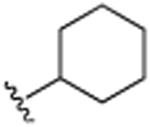
|
H | N(CH3)2 | H | 6.7 (±0.2) |
| 30 | CH3 |
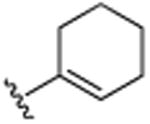
|
H | CH(CH3)2 | H | 7.8 (±0.1) |
| 31 | H | C(CH3)3 | H | N(CH3)2 | H | >25.0 |
| 32 | (CH2)3CH3 | H | H | N(CH3)2 | H | >25.0 |
| 33 | CH2CH3 | CH2CH3 | H | N(CH3)2 | H | 18.7 (±1.0) |
| 34 | CH3 |
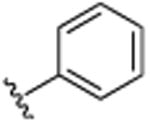
|
H | N(CH3)2 | H | 10.0 (±0.6) |
| 35 |
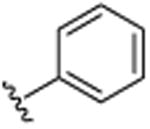
|
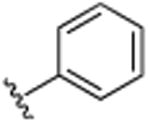
|
H | N(CH3)2 | H | >25 |
| 36 | CH3 |
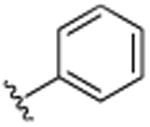
|
H | (CH2)3CH3 | H | 21.6 (±1.1) |
| 37 | CH3 |
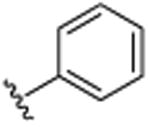
|
H | CH(CH3)2 | H | >25.0 |
| 38 | CH3 |
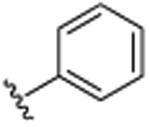
|
H | Br | H | >25.0 |
