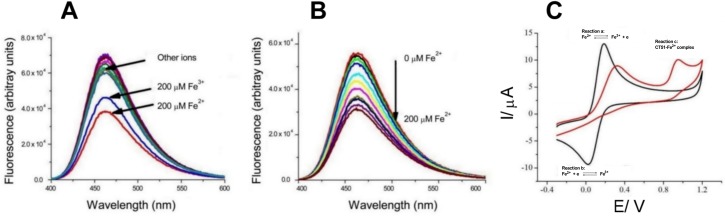
Fig 2. Cation selectivity of CT51.
(A) Fluorescence emission spectra of CT51 (20 μM) recorded before and after addition of several different chloride salts of Hg2+, Fe3+, Fe2+, Co2+, Cu2+, Ca2+, Zn2+, Mn2+, Mg2+, Ni2+, Pb2+ or Cd2+; all salt solutions (200 μM) were prepared in 20 mM HEPES buffer, pH 7.4. (B) Emission spectra of CT51 (20 μM) were recorded (λex = 330 nm) upon addition of increasing concentrations (up to 200 μM) of Fe(NH4)2(SO4)2·6H2O prepared in 20 mM HEPES buffer, pH 7.4. (C) Cyclic voltammetric analysis. The voltammograms represent the behavior of solutions containing 0.58 mM Fe2+ without CT51 (black line), or in the presence of 0.96 mM CT51 (red line). The voltammograms were performed at a scan rate of 100 mV s-1, using a potentiostat DropSens μStat 400.