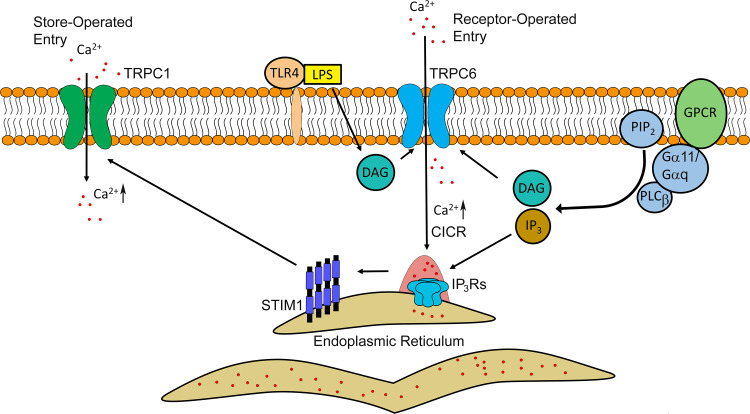
Fig. 2.
Model of receptor-mediated signaling in activating Ca2+ release and entry in endothelial cells during inflammation. The signal transduction begins with the binding of the ligand (hormone, cytokine, etc.) to specific G-protein coupled receptor (GPCR) at the surface of endothelial cells. Initial Gα11/Gαq-coupled activation of phospholipase C (PLC) β directs cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into the two secondary messengers, diacylglycerol (DAG) and inositol trisphosphate (IP3). IP3 induces clustering and activation of IP3Rs on the ER membrane, whereas DAG instigates Ca2+ entry through transient receptor potential cation channels member 6 (TRPC6) located at the plasma membrane. The latter might contribute to increases in cytosolic Ca2+ concentration igniting global Ca2+ release through IP3Rs. Depletion of the ER Ca2+ stores triggers oligomerization of stromal interaction molecule 1 (STIM1) which is required for activation of TRPC1 channel and Ca2+ entry from extracellular space. Lipopolysaccharide (LPS) also can activate Ca2+ entry through the TRPC6 channel by binding to toll-like receptor 4 (TLR4)