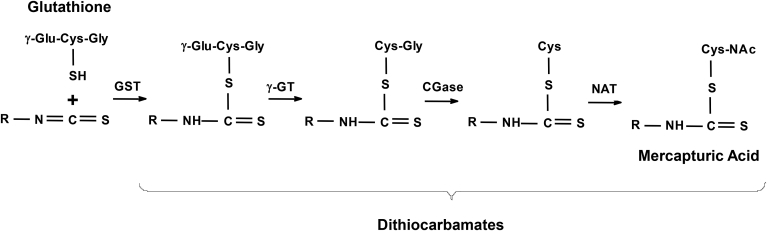
Fig. 2.
Metabolism of isothiocyanates in mammalian cells. The central carbon of the isothiocyanate (—N C S) group is electrophilic and reacts readily with sulfur-, nitrogen-, and oxygen-centered nucleophiles. The most common reaction in mammalian cells is conjugation with sulfhydryl groups, such as the sulfhydryl group of cysteine in proteins and glutathione. The reaction with glutathione is catalyzed by glutathione S-transferases (GSTs), and the resulting product is cleaved sequentially by γ-glutamyl-transpeptidase (γ-GT), cysteinyl-glycinease (GCase), and N-acetyltransferase (NAT) to give the N-acetylcysteine conjugate (mercapturic acid). The conjugates are collectively known as dithiocarbamates.