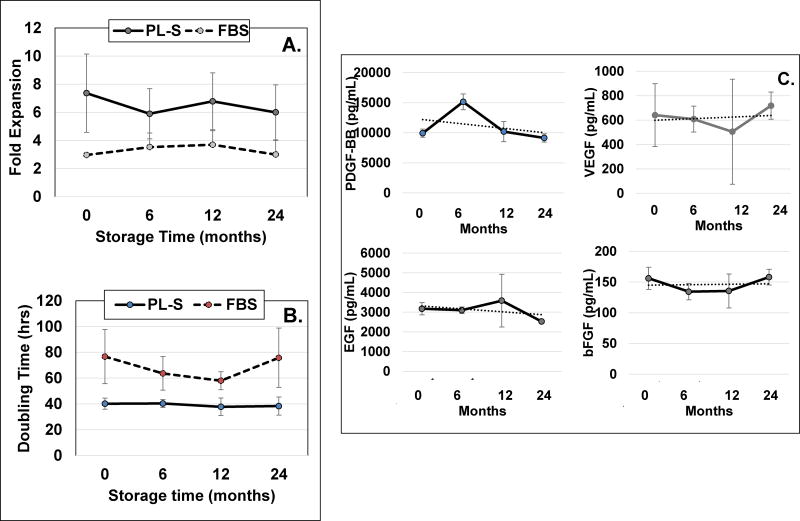
Figure 3. PL-S stability testing over a 2-year period.
Three randomly selected industrial scale lots were selected for stability testing. A) fold-expansions, and B) doubling-times were determined from each of the 3 lots of PL-S that were tested immediately after the completion of the PL-S manufacturing run (i.e. time zero) or after 1–10 mL aliquots from each PL-S lot were maintained at −80°C for 6, 12 or 24 months. A single lot of FBS was used and tested over the same time period. Using a single lot of MSC for all time points, MSCs that had been stored frozen were thawed and plated in 6 well plates that contained basal medium supplemented with either PL-S or with FBS. After cells reached confluence, they were harvested to determine fold-expansions and doubling times. C) The concentration of PDGF-BB, VEGF, EGF and bFGF in aliquots of PL-S were assessed upon completion of the PL-S manufacturing run or after being maintained at −80°C for 6, 12 or 24 months. (n=3)